Abstract
During the past few years, new immunosuppressants, such as tacrolimus, mycophenolate mofetil (MMF) and basiliximab, have been shown to successfully decrease the incidence of acute rejection, possibly acting as potent substrates for safe steroid withdrawal. Therefore, clinical outcome of 3 months steroid withdrawal, while using the above immunosuppressants, was analyzed. Clinical trial registry No. was NCT 01550445. Thirty de novo renal transplant recipients were enrolled, and prednisolone was slowly withdrawn 3 months post-transplantation by 2.5 mg at every two weeks, until 8 weeks. During steroid withdrawal, 10 patients (30.0%) discontinued the protocol and they were maintained on steroid treatment. Among 20 steroid free patients, 8 patients (40.0%) re-started the steroid within 12 months post-transplantation. By the study endpoint, 12 (40%) recipients did not take steroid and survival of patients and grafts was 100%. In conclusion, in kidney transplant patients, 3 months steroid withdrawal while taking tacrolimus, basiliximab and mycophenolate mofetil was not associated with increased mortality or graft loss. Despite various causes of failure of steroid withdrawal during the follow-up period, it is a strategy well advised for kidney transplant recipients with regard to long-term steroid-related complications.
Although corticosteroids have played an important part in the maintenance of immunosuppression after kidney transplantation, they are associated with debilitating adverse effects, including diabetes, osteoporosis, cataracts, hypertension, hyperlipidemia, obesity, avascular necrosis, mood and appearance changes and growth retardation in children (1). The treatment of these steroid-related side effects adds to the cost of transplants. In addition, such side effects increase post-transplant non-compliance, which is associated with increased incidence of acute rejection, chronic allograft nephropathy and graft loss. Given the fact that patients' death with functioning graft was an important cause of graft loss after kidney transplantation (accounting for 42.5% of graft losses in one registry analysis), and that the leading cause of death with functioning graft was cardiovascular disease, cardiovascular risk factors appear to be the most important challenge to further improving the longevity of patients with successful renal transplants (2). Many of risk factors for cardiovascular diseases overlap with the side effects of corticosteroids, such as diabetes, hypertension, hyperlipidemia and weight gain. In order to increase the patient survival after kidney transplantation, it is imperative to avoid or at least reduce such risk factors, as well as reduce the use of corticosteroids, without increasing the incidence of acute rejection of the kidney graft.
During the past few years, new immunosuppressants, such as tacrolimus, mycophenolate mofetil (MMF) and basiliximab, have been proven to decrease the incidence of acute rejection, possibly acting as potent substrates for safe steroid-free immunosuppression or steroid withdrawal. After transplantation, the major concern with any immunosuppression withdrawal protocol is the increased risk of acute and chronic rejection, potentially leading to graft damage and dysfunction or loss. Therefore, the goal of steroid withdrawal protocols is to eliminate steroid-related side-effects, while not increasing the rates of acute rejection or chronic graft loss. Development of more effective immunosuppressive agents has led to renewed attempts at steroid withdrawal after kidney transplantation (3). One of meta-analyses about the steroid withdrawal protocols applied between 7 days and 12 months post-transplantation reported that the incidence of acute rejection was increased; however, the graft and patient survival was not altered, and there was marked reduction in cardiovascular risk factors (4). Although there are many controversies about the clinical outcome after steroid avoidance or withdrawal, it is generally associated with higher rate of steroid-sensitive acute rejections, while avoiding steroid-related adverse effects. In regards with the interval between the steroid withdrawal and the transplantation, it is generally recognized that earlier steroid avoidance or withdrawal after transplantation is associated with higher rate of acute graft rejection and with lower rate of steroid-related adverse effects for the patient. Another recent meta-analysis demonstrated that steroid withdrawal performed between 3 and 6 months after renal transplantation was found to increase the incidence of acute rejection in patients on cyclosporine, but not tacrolimus, whereas no impact on graft and patient survival was reported (5).
In the present study, we analyzed clinical outcomes of kidney transplantation recipients on steroid withdrawal protocol applied 3 months after renal transplantation, using tacrolimus, MMF and basiliximab, and also compared clinical characteristics of patients in whom corticosteroids were successfully withdrawn with those of patients in whom corticosteroids had to be reinitiated because of acute rejection or other problems.
This study was an open-label, prospective, controlled clinical trial at a single center, with withdrawal of steroid at 3 months after renal transplantation. In this 12-month study, 30 de novo renal transplant recipients who gave written informed consent were enrolled.
Patients older than 13 yr who received primary kidney transplant from a deceased or living donor were eligible for the study. The exclusion criteria comprised receipt of a kidney from a donor with positive lymphocyte cross-match, an ABO incompatible donor, current hepatitis B or C, HIV positive, neutrophil count less than 500/µL, WBC count less than 1,000/µL, hemoglobin less than 5 g/dL, platelet count less than 30,000/µL, any mental disorders, any malignant disease and pregnant or lactating recipients.
Tacrolimus (Tacrobell®, Chong Kun Dang, Seoul, Korea) was initiated one day prior to transplantation, at 0.1 mg/kg orally twice a day. The target trough blood levels of tacrolimus were 10-15 ng/mL within 3 months post-transplantation and 5-10 ng/mL thereafter. MMF was given at a dose of 1 to 2 g/day. All recipients received 20 mg of basiliximab just prior to transplant and 4 days after transplantation. In the immunosuppressive protocol, methyl-prednisolone was injected with the following doses; 500 mg on the day of operation, 250 mg on the day after, 125 mg on day 2, and 60 mg on day 3. Prednisolone given at a dose of 30 mg/day on day 4 was slowly tapered to the maintenance dose of 10 mg/day by the end of the first month. The dose of 10 mg prednisolone a day was maintained until 3 months after kidney transplantation.
Those patients who fulfilled the entry criteria were enrolled in this prospective controlled trial of steroid withdrawal 3 months after transplantation. The entry criteria were, as follows: 1) no episode of clinically treated or biopsy confirmed acute rejection prior to study entry; 2) serum creatinine level equal to or less than 2 mg/mL on 3 separate measurements; 3) no proteinuria (i.e., urine protein less than 1,000 mg/24 hr); 4) tacrolimus trough level > 5 ng/mL, without signs of nephrotoxicity; and 5) agreement to the projected protocol follow-up.
For the patients who entered the protocol, prednisolone was slowly withdrawn by 2.5 mg every 2 weeks, until 8 weeks after entering the protocol (i.e., 5 months post-transplantation). The patients visited the center every week and were closely monitored for symptoms and signs, blood cell count, chemistry and urinalysis.
Study visits took place at baseline before transplantation, every day until 2 weeks post-transplantation, then every week until 5 months post-transplantation, including regular monitoring of vital signs, adverse events, serious adverse events, hematology and blood chemistry. At 6 months post-transplantation, urine samples for 24 hr were collected from all recipients to analyze the creatinine clearance and urinary protein excretion; urine and serum were both checked for BK (polyoma) virus using PCR. Biopsy was performed in cases of deteriorating graft function without any obvious pre- or post-renal causes. Biopsy samples were examined by the pathology specialist and were classified in agreement with the 1997 Banff criteria.
The primary efficacy variable was the incidence of biopsy-confirmed acute rejection. Secondary efficacy variables included the cumulative incidence of composite endpoint of death and graft loss; patient and graft survival at month 12; and the percentage of patients free of steroids at month 6 and 12. Safety variables included the incidence of adverse events and serious adverse events, blood pressure, lipid levels (total cholesterol), blood urea nitrogen (BUN) and blood glucose levels.
Statistical analyses were performed using Statistical Package for the Social Services (SPSS version 11.0.1) (SPSS Inc., Chicago, IL, USA). Student's t-test was used to compare continuous variables of patient characteristics and chi-square test was used to determine categorical demographic variables and outcome differences. Continuous variables are expressed as mean ± standard deviation (SD). At 6 and 12 months, the actual patient and graft survival were reported as life-table estimates. Initiation of maintenance steroids at any time after 3 months post-transplantation was considered as failure of treatment.
The study was conducted in full compliance with the amended Declaration of Helsinki, following approval from the institutional review board of Ajou University Hospital (AJIRB-CRO-08-067). All of the 30 subjects enrolled in this study gave written informed consents for not only for the transplant surgery but also for this clinical trial prior to kidney transplantation.
Between May 2008 and October 2009, 30 patients were enrolled in the study. Study sample is summarized in Table 1. Twenty-two patients (73.3%) received kidneys from deceased donors and 8 patients (26.7%) from living donors. At 3 months post-transplantation, only 3 patients (10%) discontinued the study prematurely because of serum creatinine level higher than 2.0 mg/dL, without an episode of rejection, and 27 patients (90%) continuously followed the steroid withdrawal protocol and were included in the intent to treat (ITT) group in the study.
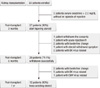
During steroid withdrawal, between 3 and 5 months post-transplantation, 7 patients (26.0%) discontinued the protocol and were maintained on steroid treatment by the following reasons: 1 patient withdrew the written consent, 1 patient with acute rejection Banff IA, 2 patients with borderline changes on the biopsy, 1 patient with steroid withdrawal symptoms and 2 patients with BK virus PCR positive. Among 20 patients with steroid free immunosuppression with tacolimus and MMF, 8 patients (40.0%) re-started the steroid for the following reasons: 2 patients with borderline changes on the biopsy, 5 patients with BK virus PCR positive, and 1 patient with CMV disease within a year post-transplantation (Fig. 1). The overall causes of failure for steroid withdrawal within 1 year post-transplantation are summarized in Table 2.
Within 3 months post-transplantation, none of the patients had acute rejection. During the steroid withdrawal, between 3 and 6 months post-transplantation, 1 patient (3.7% of ITT group) had biopsy-confirmed acute rejection (Banff IA) and 2 patients (7.4%) had borderline change on the biopsy. Two out of 20 completely steroid-free patients (10.0%) had borderline change on the graft biopsy within 1 yr post-transplantation. All patients with acute rejection or borderline change required re-introduction of oral steroid, without steroid pulse therapy, and responded to the steroid re-introduction.
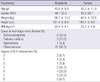
At 12 months after transplantation, survival of the patients and grafts was 100%. Serum creatinine levels of 30 patients at 3 months, 6 months, and 12 months post-transplantation were 1.33 ± 0.43 mg/dL, 1.47 ± 0.44 mg/dL and 1.55 ± 0.49 mg/dL, respectively. Estimated GFR by MDRD (Modification of Diet in Renal Disease) at 3 months, 6 months, and 12 months post-transplantation were 58.9 ± 16.4 mL/min, 53.2 ± 17.9 mL/min, and 49.7 ± 16.0 mL/min, respectively. At 12 months post-transplantation, among 27 ITT patients, the serum creatinine levels of 12 cases in the withdrawn group were not statistically different from those of 15 cases in the steroid re-start group (1.35 ± 0.18 mg/dL vs 1.57 ± 0.54 mg/dL, P = 0.197). The estimated GFR at 12 months post-transplantation was higher in the withdrawn group than in the re-start group (58.8 ± 11.1 mL/min/1.73 m2, vs 46.5 ± 16.1 mL/min/1.73 m2, P = 0.033) (Table 3).
Table 3 shows the physiologic variables, laboratory values and immunosuppression in the investigated groups. There were no significant differences between the groups in systolic blood pressure, diastolic blood pressure, mean blood pressure, body weight, WBC count, hemoglobin, platelet count, serum creatinine and serum glucose (P > 0.05, independent t-test). Total cholesterol in the steroid withdrawn group was 158.0 ± 50.3 mg/dL, which was significantly lower than that of the steroid re-start group 186.8 ± 34.6 mg/dL (P = 0.048, independent t-test). Similarly, BUN in the steroid withdrawn group was 18.8 ± 4.8 mg/dL, which was also significantly lower than that of the steroid re-start group 24.2 ± 6.9 mg/dL (P = 0.034, independent t-test). The serial changes of mean cholesterol and mean BUN at 3 months, 6 months, and 12 months post-transplantation in the different groups are shown in Fig. 2.
Adverse events in the ITT patients between 3 and 12 months post-transplantation (i.e. beyond the starting point of steroid withdrawal) were, as follows: 8 cases of alopecia, 7 cases of upper respiratory infection, 11 cases of gastrointestinal symptoms, 4 cases of leukocytopenia ( < 3,000/µL), 2 cases of transient headache, 2 cases of oral ulcer and 1 case of fatigue. serious adverse events (SAEs), which required to maintain the dose of steroid or needed either to discontinue or decrease the dose of MMF, were as follows: 5 cases of acute rejection or borderline change on biopsy, 7 cases of urinary BK virus PCR-positive, 1 case of CMV, and 1 case of severe steroid withdrawal symptoms, as shown in Fig. 1.
Until recently, corticosteroids had been the mainstay of kidney transplant immunosuppressive protocols. However, well-known side effects of corticosteroids have increased the interest in steroid-withdrawal immunosuppression for kidney transplant recipients, and the goal of steroid minimization protocols has been to eliminate or minimize steroid-related side-effects, while not increasing the rates of acute rejection or graft loss. It is widely believed that the safety of steroid-free immunosuppression has improved the quality of life of kidney recipients since the mid-1990s, with the availability of tacrolimus and MMF and especially, with the regular use of induction antibody therapy.
Hricik (6) showed that withdrawal of steroid in early phase after renal allograft transplantation was dangerous and could lead to clinical acute rejection with an incidence of 30% to 50%. With the development of many immunosuppressants, such as tacrolimus, MMF and basilixumab, some regimens have been demonstrated to be effective (7-9). Our present study demonstrated that one patient (3.7% of ITT population) had an episode of biopsy-confirmed acute rejection (Banff IA) and 4 patients (14.8% of ITT population) had borderline changes on the graft biopsy within 1 yr post-transplantation. Five patients with acute rejection or borderline change were treated easily with oral dose of steroid and were maintained on steroid during the follow-up period. Given the fact that only 5 patients (16.7% of enrolled subjects) had either biopsy-confirmed acute rejection or borderline change on the graft biopsy, the incidence of acute rejection within a year after transplantation was lower than that in previous reports. Furthermore, the fact that neither patient death nor graft loss occurred within 1 yr post-transplantation in our study population strongly suggests that steroid can safely be withdrawn from tacrolimus, basilixumab and MMF-treated renal allograft recipients. The reason that steroid can be withdrawn could partly be explained by the special relationship between FK binding protein 52 (FKBP-52) and glucocorticoid receptor compound (GCR) (10). The incidence of acute renal transplant rejection has dramatically decreased over the past decades, with most centers reporting incidence rates between 10% and 20% (11). After trial or completion of steroid withdrawal, the overall incidence of acute rejection was 16.7%, which was not higher than that of the kidney transplant population on steroids. Therefore, the strategy of steroid withdrawal could not increase the incidence of acute rejection.
Previously, one study demonstrated that 33% of kidney recipients had urinary BK virus PCR positivity, with or without clinical manifestations, such as positive Decoy cell and decrease of graft function (12). Our data showed that 23.3% of the study population had urinary BK virus PCR positivity either during or after steroid withdrawal. Therefore, in terms of BK virus nephropathy, the strategy of steroid withdrawal could not increase the incidence of urinary BK virus PCR positivity.
The lowering of cholesterol levels has been reported to be one of the benefits resulting from the withdrawal of steroid therapy (13, 14). Furthermore, reduction of blood pressure has also been reported (15), albeit not unanimously (13, 14). Even though we were unable to demonstrate any reduction of blood pressure in our patient population, steroid withdrawal immunosuppression significantly reduced total cholesterol level at 1 yr post-transplantation. Noteworthy, our finding that steroid withdrawal immunosuppression significantly reduced the blood urea nitrogen (BUN) at 1 yr post-transplantation could be comparable to the previous report that corticosteroid could disproportionally increase BUN levels (16). The study results demonstrated that the mean eGFR of the steroid withdrawn group was significantly higher than that of the other group. This mainly reflected the fact that the subject population of the steroid withdrawn group consisted of patients who underwent the uneventful course after kidney transplantation, whereas the other group included patients with acute rejection, BK virus nephropathy or CMV infection.
One of the limitations of our study was that it was a single-center analysis. The pros and cons of single-center versus multicenter reports are well known. Nevertheless, our study has the advantage of being coordinated by a small group of people with well-defined decision-making protocol and patient care algorithm. Because we applied our protocol to 30 transplant recipients, additional studies in larger number of kidney recipients should be necessary. The second limitation was that we did not perform protocol biopsies. Although mean creatinine levels remained unchanged in our patient population, there was the possibility that we failed to observe some progressive fibrosis due to the absence of prednisone maintenance therapy. The third limitation was that our study was not randomized, because we started with a pilot study. However, in accordance with our present findings, the 12-month results of a prospective randomized study (17), in which 364 kidney transplant recipients (on tacrolimus and MMF) received either two doses of anti-IL-2 receptor antibody (daclizumab) plus 3 days of prednisone or steroids for 16 weeks, found no difference in the incidence of biopsy-proven acute rejection between the two groups (15% in the dacrlizumab group vs 14% in the control group) (17). The fourth limitation was that long-term benefits of steroid withdrawal were not assessed with regard to well documented complications, such as cardiovascular disease and osteoporosis, because it was beyond the scope of our study.
In conclusion, this study confirmed that steroid withdrawal at 3 months post-transplantation, while using tacrolimus, basiliximab and MMF in kidney transplantation, was not associated with increased mortality or graft loss. In spite of various causes of failure of steroid withdrawal during the follow-up period, steroid withdrawal after 3 months post-transplantation is a strategy well advised for kidney transplant recipients, because it could provide beneficial effects on short-term steroid-related complications, such as increased cholesterol and uremia and, possibly, on long-term steroid-related complications.
Figures and Tables
References
1. Veenstra DL, Best JH, Hornberger J, Sullivan SD, Hricik DE. Incidence and long-term cost of steroid-related side effects after renal transplantation. Am J Kidney Dis. 1999. 33:829–839.
2. Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000. 57:307–313.
3. Matas AJ. Steroid elimination-who, when, how? Transplant Proc. 2008. 40:S52–S56.
4. Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation. 2010. 89:1–14.
5. Pascual J, Galeano C, Royuela A, Zamora J. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation. 2010. 90:343–349.
6. Hricik DE. Steroid withdrawal in renal transplant recipients: pro point of view. Transplant Proc. 1998. 30:1380–1382.
7. Wlodarczyk Z, Walaszewski J, Perner F, Vitko S, Ostrowski M, Bachleda P, Kokot F, Klinger M, Szenohradszky P, Studenik P, Navratil P, Asztalos L, Rutkowski B, Kalmar KN, Hickey D. Steroid withdrawal at 3 months after kidney transplantation: a comparison of two tacrolimus-based regimens. Transpl Int. 2005. 18:157–162.
8. Kato Y, Tojimbara T, Iwadoh K, Koyama I, Nanmoku K, Kai K, Sannomiya A, Nakajima I, Fuchinoue S, Teraoka S. Early steroid withdrawal protocol with basiliximab, cyclosporine and mycophenolate mofetil in renal-transplant recipients. Int Immunopharmacol. 2006. 6:1984–1992.
9. Lee YJ, Kim B, Lee JE, Kim YG, Kim DJ, Kim SJ, Joh JW, Oh HY, Huh W. Randomized trial of cyclosporine and tacrolimus therapy with steroid withdrawal in living-donor renal transplantation: 5-year follow-up. Transpl Int. 2010. 23:147–154.
10. Prima V, Depoix C, Masselot B, Formstecher P, Lefebvre P. Alteration of the glucocorticoid receptor subcellular localization by non steroidal compounds. J Steroid Biochem Mol Biol. 2000. 72:1–12.
11. Bemelman FJ, de Maar EF, Press RR, van Kan HJ, ten Berge IJ, Homan van der Heide JJ, de Fijter HW. Minimization of maintenance immunosuppression early after renal transplantation: an interim analysis. Transplantation. 2009. 88:421–428.
12. Vera-Sempere FJ, Rubio L, Moreno-Baylach MJ, García A, Prieto M, Camañas A, Mayordomo F, Sánchez-Plumed J, Beneyto I, Ramos D, Zamora I, Simón J. Polymerase chain reaction detection of BK virus and monitoring of BK nephropathy in renal transplant recipients at the University Hospital La Fe. Transplant Proc. 2005. 37:3770–3773.
13. Hollander AA, Hene RJ, Hermans J, van Es LA, van der Woude FJ. Late prednisone withdrawal in cyclosporine-treated kidney transplant patients: a randomized study. J Am Soc Nephrol. 1997. 8:294–301.
14. Matl I, Lácha J, Lodererová A, Símová M, Teplan V, Lánská V, Vítko S. Withdrawal of steroids from triple-drug therapy in kidney transplant patients. Nephrol Dial Transplant. 2000. 15:1041–1045.
15. Kupin W, Venkat KK, Goggins M, Douzdjian V, Escobar F 3rd, Mozes M, Abouljoud M. Improved outcome of steroid withdrawal in mycophenolate mofetil-treated primary cadaveric renal transplant recipients. Transplant Proc. 1999. 31:1131–1132.
16. Feinfeld DA, Bargouthi H, Niaz Q, Carvounis CP. Massive and disproportionate elevation of blood urea nitrogen in acute azotemia. Int Urol Nephrol. 2002. 34:143–145.
17. ter Meulen CG, van Riemsdijk I, Hené RJ, Christiaans MH, Borm GF, van Gelder T, Hilbrands LB, Weimar W, Hoitsma AJ. Steroid-withdrawal at 3 days after renal transplantation with anti-IL-2 receptor alpha therapy: a prospective, randomized, multicenter study. Am J Transplant. 2004. 4:803–810.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download