Abstract
Glycosaminoglycans are important structural components in the skin and exist as various proteoglycan forms, except hyaluronic acid. Heparan sulfate (HS), one of the glycosaminoglycans, is composed of repeated disaccharide units, which are glucuronic acids linked to an N-acetyl-glucosamine or its sulfated forms. To investigate acute ultraviolet (UV)-induced changes of HS and HS proteoglycans (HSPGs), changes in levels of HS and several HSPGs in male human buttock skin were examined by immunohistochemistry and real-time quantitative polymerase chain reaction (qPCR) after 2 minimal erythema doses (MED) of UV irradiation (each n = 4-7). HS staining revealed that 2 MED of UV irradiation increased its expression, and staining for perlecan, syndecan-1, syndecan-4, CD44v3, and CD44 showed that UV irradiation increased their protein levels. However, analysis by real-time qPCR showed that UV irradiation did not change mRNA levels of CD44 and agrin, and decreased perlecan and syndecan-4 mRNA levels, while increased syndecan-1 mRNA level. As HS-synthesizing or -degrading enzymes, exostosin-1 and heparanase mRNA levels were increased, but exostosin-2 was decreased by UV irradiation. UV-induced matrix metalloproteinase-1 expression was confirmed for proper experimental conditions. Acute UV irradiation increases HS and HSPG levels in human skin, but their increase may not be mediated through their transcriptional regulation.
Glycosaminoglycans (GAGs) are long, linear polysaccharide chains composed of repeated specific disaccharide units and are known to have structural, space-filling, and physiological functions in the skin (1). Six types of GAGs have been found, including chondroitin sulfate, dermatan sulfate, keratan sulfate, heparan sulfate (HS), heparin, and hyaluronic acid. They exist as proteoglycan forms, except hyaluronic acid (1, 2). Due to their highly negatively-charged properties, GAGs may contribute to tissue water holding capacities and maintenance of water content (1, 3).
HS is composed of repeated disaccharide units, glucuronic acids linked to an N-acetyl-glucosamine or its sulfated forms and is known to be involved in various physiological functions, including cell-matrix adhesion, cell proliferation, growth factor or cytokine binding, immune cell invasion, and cellular signaling as a part of various heparan sulfate proteoglycans (HSPGs) (4-6). HSPGs, such as perlecan, agrin, and collagen XVIII, exist in basement membranes and function both as an immune cell invasion barrier and modulators of local growth factor or cytokine gradients by their binding to HS (4). Other HSPGs, such as syndecans, glypicans, and exon v3-containing isoforms of CD44 (CD44v3), exist on the cell plasma membrane and participate in cell adhesion, migration, and cellular signaling (5-8).
Ultraviolet (UV) irradiation is known to be a major cause of extrinsic skin aging (photoaging); accumulation of UV irradiation-induced damage leads to induction of matrix metalloproteinases (MMPs) and reduction of type I collagen production which may result in skin photoaging (9, 10). Of the GAGs, hyaluronic acid is reported to be increased by acute UV irradiation in the epidermis but decreased in the dermis despite hyaluronic acid synthase induction (11). However, hyaluronic acid was reported to show no significant change after 4-week UVB irradiation, while chondroitin sulfate was increased after 4-week UVB irradiation and after single-dose irradiation of UVB (12). In another report, the hyaluronic acid content ratio of photoaged/intrinsically-aged skin was increased in aged skin compared to young skin, and the total dermal sulfated GAG content ratio of photoaged/intrinsically-aged skin was also increased (3). Recently, UV-induced decreases of various proteoglycan transcriptional levels in human dermal fibroblasts were reported (13), while serglycin was reported to be increased by UVB irradiation (12); however, sufficient evidence for UV-induced changes of various proteoglycans has not yet found.
Therefore, in this study, we investigated UV-induced changes of HS and HSPGs by immunohistochemistry and measuring mRNA levels in human buttock skin in vivo.
Healthy young Korean male volunteers (21-40 yr, 28.9 ± 4.6 yr [mean ± standard deviation], n = 23), provided normal or 2 minimal erythema doses (MED) UV-irradiated buttock skin samples. UV irradiation was performed as previously described (14). Briefly, UV irradiation to the buttock skin was performed with a F75/85W/UV21 fluorescent lamp with an emission spectrum between 275 and 380 nm (peak at 310-315 nm). A Kodacel filter (TA401/407; Kodak, Rochester, NY, USA) was used to remove UV-C (< 290 nm). UV strength was measured using a Waldmann UV meter (model 585100, Waldmann, Villingen-Schwenningen, Germany). The MED was determined at 24 hr after irradiation. Potential volunteers were excluded if they had sunlight hypersensitivity or a skin disease such as atopic dermatitis or psoriasis, if they had suffered from other acute or severe diseases, or if they had undergone recent treatment with any medicine that might affect skin homeostasis, including retinoid or steroids. Skin specimens were obtained at 0, 24, 48, or 74 hr after UV irradiation by punch biopsy from all subjects, who gave written informed consent in accordance with the principles of the Declaration of Helsinki.
Immunohistochemical staining was performed as previously described (2). Briefly, 4 µm sections from the paraffin blocks of buttock skin specimens were dewaxed in xylene, rehydrated in graded alcohol, and washed with distilled water. For frozen tissues, 4 µm sections were fixed in acetone for 5 min at -20℃. Endogenous peroxidase activity quenching was performed for 6 min with 3% hydrogen peroxide solution. After blocking for 30 min with blocking solution from SPlink HRP Broad Bulk Kit (Golden Bridge International, Mukilteo, WA, USA), the sections were incubated with primary antibodies (Table 1) in a humidified chamber for 18 hr at 4℃, incubated with biotinylated secondary antibodies for 15 min, and incubated with streptavidinhorseradish peroxidase conjugate for 15 min (Golden Bridge International). For perlecan, Ultra V Block (Thermo Scientific, Fremont, CA, USA) and biotinylated goat anti-rat IgG (Vector Laboratories Inc., Burlingame, CA, USA) were used as the blocking reagent and secondary antibody, respectively. The sections were developed using AEC (3-amino-9-ethylcarbazole, Golden Bridge International) for 3 to 5 min depending on types of antibodies and mounted with Faramount medium (DAKO, Carpinteria, CA, USA). Cell nuclei were not counterstained. Photos of sections were obtained using a Leica DFC280 camera (Leica, Heerbrugg, Switzerland) with an Olympus BX51 microscope (Olympus, Tokyo, Japan).
After separation of the epidermis from the dermis by 3 min incubation in 55℃ phosphate-buffered saline, total RNA was extracted from the epidermis tissues using the Trizol method (Invitrogen, Carlsbad, CA, USA), and 1 µg of total RNA was converted to cDNA using a First Strand cDNA Synthesis Kit (MBI Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions (14). To quantitatively estimate the mRNA expression of target genes, PCR was performed in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with iQ SYBR Green Supermix (Bio-Rad), according to the manufacturer's instructions, using the following primer pairs: perlecan (forward, 5'-ccccacaccatcacctggta-3'; reverse, 5'-ccgttactgacgtgacacac-3'), CD44 (forward, 5'-ttcttcgacccaatctcaca-3'; reverse, 5'-tgaaagtggtcctgtcctgt-3'), syndecan-1 (forward, 5'-ctctgtgccttcgtctttcc-3'; reverse, 5'-ccaccttcctttgccattta-3'), syndecan-4 (forward, 5'-tgggtggttgagtgagtgaa-3'; reverse, 5'-agcctgaagaaagcaaacca-3'), agrin (forward, 5'-aacctgtgccgatgagaaga-3'; reverse, 5'-ggagaagccgttgaagtcag-3'), MMP-1 (forward, 5'-aagcgtgtgacagtaagcta-3'; reverse, 5'-aaccggacttcatctctg-3'), exostosin-1 (forward, 5'-tcatcagcagagccagattg-3'; reverse, 5'-cacagaagccagtgaggtga-3'), exostosin-2 (forward, 5'-gagtgcatcaacaagtttgc-3'; reverse, 5'-atgttggggaagctcttcag-3'), heparanase (forward, 5'-ttttccaggtggttgagagc-3'; reverse, 5'-cccaatttatccagccacat-3'), and 36B4 (forward, 5'-tcgacaatggcagcatctac-3'; reverse, 5'-tgatgcaacagttgggtagc-3'). The PCR cycling conditions were 50℃ for 2 min and 95℃ for 2 min, followed by 40 cycles at 95℃ for 22 sec and 60℃ for 1 min. Data were analyzed using the comparative Ct method, normalized to 36B4 and presented as mean relative fold changes ± standard deviation. Statistical analyses were performed using the Wilcoxon signed-rank test. P values of less than 0.05 were considered statistically significant.
For investigation of acute UV irradiation-dependent changes of HS and HSPGs in the human skin, irradiated young male buttock skin was analyzed with immunohistochemistry. HS staining (n = 4, 26.3 ± 4.5 yr) was observed weakly in dermal cells and epidermal keratinocytes and strongly seen in the basement membrane zone and dermal blood vessels (Fig. 1A). After UV irradiation, a representative epidermal HS staining began to increase from 24 hr, peaked at 48 hr, and still remained at 72 hr after irradiation (Fig. 1A). HS staining in the dermal-epidermal junction tended to slightly increase, and staining in the papillary dermis was increased at 48 and 72 hr after irradiation (Fig. 1A). There were individuals who showed further increase at 72 hr. This result suggests that UV irradiation may induce expression levels of HSPGs in human skin. Therefore, we investigated UV-induced changes of several HSPGs, including perlecan, sydecan-1 and -4, and CD44 isoforms containing exon v3 (CD44v3).
Perlecan, a basement membrane HSPG, stained strongly in the dermal-epidermal junction and blood vessels and weakly in the papillary dermis and epidermis (n = 7, 30.6 ± 5.3 yr) (Fig. 1B). A representative perlecan staining in the dermal-epidermal junction, papillary dermis, and epidermis was increased at 24 or 48 hr after UV irradiation, and it seemed to be recovered at 72 hr (Fig. 1B). There were individuals who showed further increase at 72 hr. Syndecan-1 (n = 4, 26.3 ± 4.5 yr) and -4 (n = 4, 26.3 ± 4.5 yr) are plasma membrane-type HSPGs, and their expression was strong in epidermal and dermal cells. UV irradiation markedly increased their epidermal staining at 24 to 72 hr after irradiation (Fig. 1C, D), peaked at 48 or 72 hr depending on the individual. CD44v3, CD44 isoforms containing HS chain, stained strongly in the epidermis and faintly in dermis; CD44v3 staining was similarly increased by UV at 24 to 72 hr after irradiation, peaked at 24 or 48 hr depending on the individual (n = 5, 29.2 ± 3.6 yr; Fig. 1E). Staining of the CD44 standard form, CD44s, which lacks all variant exons (7, 15), was also increased by UV irradiation in a similar pattern as CD44v3 staining, peaked at 24 or 48 hr depending on the individual, though its epidermal expression was higher in basal layer cells, and its dermal stain was stronger than that of CD44v3 (n = 5, 27.8 ± 3.6 yr; Fig. 1F). MMP-1 staining was performed to confirm proper UV irradiation (n = 6, 30.2 ± 4.3 yr). While faint MMP-1 staining was observed in non-irradiated skin (0 hr); strong epidermal and moderate dermal MMP-1 staining was seen after UV irradiation (Fig. 1G), as previously reported (9, 16). MMP-1 staining was peaked at 24, 48, or 72 hr depending on the individual. These results suggest that acute UV irradiation may increase HSPG protein-level expression in human skin, which results in an increase of HS staining in UV-irradiated skin tissue.
To investigate whether the increase of HSPG level by UV irradiation is mediated by induction of HSPG transcription, mRNA expression levels of several HSPGs, including perlecan, CD44, syndecan-1 and -4, and agrin, were measured in UV-irradiated human buttock skin samples using real-time qPCR (n = 6, 27.5 ± 3.0 yr).
Unexpectedly, perlecan mRNA levels at 24 and 48 hr after UV irradiation were significantly decreased, compared to that at 0 hr (Fig. 2A), while perlecan staining was increased by UV irradiation (Fig. 1B). In contrast, syndecan-1 mRNA levels were increased (Fig. 2B), like its immunohistochemical stain (Fig. 1C), but mRNA levels of syndecan-4 showed a significant decrease after UV irradiation (Fig. 2C) in spite of its increased protein staining (Fig. 1D). The mRNA levels of CD44 and agrin, another basement membrane HSPG (4), did not show significant changes by UV irradiation (Fig. 2D, E). The MMP-1 mRNA expression level was very low at 0 hr and markedly increased at 24 hr after UV irradiation (Fig. 2F), as previously reported (9, 16). These results suggest that the increase of various HSPG protein levels in human skin by UV irradiation may not be mediated by induction of their mRNA transcriptional levels, except for syndecan-1.
To investigate the transcriptional changes of HS synthesizing or degrading enzymes by UV irradiation, mRNA expression levels of HS-chain synthesizing enzymes, exostosin-1 and -2, and a HS degrading enzyme, heparanase, were further analyzed in UV-irradiated human buttock skin samples using real-time qPCR.
Exostosin-1 mRNA level at 24 hr after UV irradiation were significantly increased (Fig. 3A), while exostosin-1 mRNA levels at 48 and 72 hr were significantly decreased by UV irradiation (Fig. 3B). Heparanase mRNA levels were also significantly increased by UV irradiation, from 24 to 72 hr (Fig. 3C), similar to the previous report (17). These results suggest that UV irradiation may increase both synthesis and degradation of HS chain through upregulation of responsible enzymes.
In this study, we tried to examine the changes in HS and HSPG expression by acute UV irradiation in the human skin and demonstrated that the staining of HS and HSPGs, including perlecan, syndecan-1 and -4, and CD44v3, was increased by UV irradiation. However, the mRNA levels of those HSPGs in irradiated skin were not increased by UV, except syndecan-1. These results suggest that, rather than transcriptional regulation, another critical HSPG regulatory mechanism in UV response may exist at the translational or posttranslational level.
Our previous paper reported the mRNA downregulation of most kinds of proteoglycans and GAG chain-synthesizing enzymes by UV irradiation in cultured human dermal fibroblasts (13). In that study, perlecan, agrin, and syndecan-2 mRNA expression was lowered by UV irradiation, and only syndecan-1 mRNA was increased (13), similar to our present mRNA result. This similar UV-induced mRNA regulation between cultured dermal fibroblasts and epidermis suggests that UV irradiation induces downregulation of HSPG mRNA, but their actual protein level increase should be mediated by other unknown mechanisms in human skin.
Several papers have described change in HS by acute UV irradiation and in photoaged skin. Chronically UVB-irradiated hairless mouse skin was observed to contain and produce increased HS and heparin proteoglycans (18). In contrast, Iriyama et al. (17) reported that HS staining in the dermal-epidermal junction was decreased in acute UVB-irradiated buttock skin, while perlecan core protein and total HS staining did not change, probably through induction of heparanase activity by UV irradiation. They also showed a reduction of HS staining in the dermal-epidermal junction in sun-exposed skin of elderly people, but not in sun-protected skin (17). In addition, another paper showed no significant difference of HS amount between photo-exposed skin and photo-protected skin (19). These results seem to be controversial, and especially, the results from Japanese group showed UV-induced HS decrease and no change of HSPG core proteins, in contrast to our results, although their experimental conditions were quite similar with ours. One possible cause was from the different epitope structures between 10E4 and JM403 clones, which were characterized by mixed N-acetylated/N-sulfated glucosamine-containing HS sequence and glucuronic acidrich HS sequences with N-deacetylated glucosamine, respectively, and their staining could have different patterns regulated by different sulfuring enzymes (20). The other reason was probably caused by different UV-B light sources. In our study, a fluorescent lamp with an emission spectrum from 275 to 380 nm was used with a wavelength filter to remove UV-C (< 290 nm), since UV-C is known to be completely removed by ozone layer during solar irradiation (21), but their UV-B radiation source had emission at a wavelength of 280-360 nm, and no filter was engaged (17). Because UV-C has shorter wavelength and higher energy than UV-B, involvement of small amount of UV-C may cause different results. Therefore, in our experiment, despite increase of heparanase mRNA expression, UV irradiation results in increased staining of HS, mainly through UV irradiation-induced increase of several HSPG members' protein levels.
In the present study, CD44v3 and CD44s staining was increased with no change in mRNA level. In the literature, reports have shown that CD44 was reduced by UV irradiation at early time points. In HaCaT cells, it was reported that CD44 mRNA was decreased at 6 hr and recovered within 24 hr after UV irradiation, and the soluble form of CD44 was increasingly released into cultured medium by UV irradiation (22). Additionally, in hairless mice, CD44 staining was observed to decrease at 2 hr after UV irradiation (23). These results also imply that the increase of CD44v3 or CD44s protein levels at 24 to 72 hr after UV irradiation may not be solely dependent on transcriptional regulation. Syndecan-1 and -4 showed strong epidermal expression, while syndecan-2 showed moderate expression (8); our results showed that syndecan-1 and -4 mRNA levels were higher than syndecan-2 in cultured epidermal keratinocytes and HaCaT cells while syndecan-3 had a very low level of expression as determined by real-time qPCR (our unpublished data). No report has been published on UV irradiation-induced regulation of syndecan-1 or -4 in the literature.
UV-induced downregulation or inhibition of proteases for HSPGs may be a possible posttranslational-level mechanism of our observation. Stromelysin, collagenase, and plasmin with heparanase activity were reported to have the ability to degrade perlecan (24), but they have been known to be increased by UV irradiation (9, 16, 25). Lysosomal proteases cathepsin B, L, and S are also known to have the ability to cleave perlecan (26, 27), and among them, cathepsin B expression was reported to be reduced in UVA-irradiated fibroblasts and photoaged skin (28). However, its involvement in UV-induced increase of perlecan protein level, in spite of lowered perlecan mRNA transcription, should be further examined. UV-induced regulation of cathepsin L or S has not yet been reported. Membrane-type 1 and 3 MMP, and a disintegrin and metalloproteinase 10 (ADAM10) were reported to have shedding activity of CD44 during migration (29), and their UV-induced regulation also has not yet been reported. No protease(s) for syndecan-1 or -4 has been reported.
UV effect on HS chain synthesis and degradation was also examined by measuring exostosin-1 and -2, and heparanase mRNA expression. However, since exostosin-1 and heparanase mRNA levels were both upregulated by UV irradiation, it is hard to say that synthesis or degradation seemed dominant.
Since HS can bind to various growth factors and promote cell proliferation (17), increased HS chain by acute UV irradiation may participates in UV-induced epidermal hyperplasia. Additionally, HS increase by UV acute irradiation may cause accumulation of HS in photoaged skin, at least partly supporting the increase of total sulfated GAG in photoaged skin (3).
In conclusion, acute UV irradiation increases HS and HSPG levels in human skin, but their increase may not be mediated through their transcriptional regulation.
Figures and Tables
Fig. 1
Immunohistochemistry of HS and HSPGs in human male buttock skin with acute UV irradiation. Healthy young Korean male buttock skin was irradiated with 2 MED of UV and skin specimens were obtained at 0, 24, 48, or 74 hr after UV irradiation by punch biopsy. Immunohistochemical staining (n = 4-7) for HS (A), perlecan (B), syndecan-1 (C), syndecan-4 (D), CD44v3 (E), CD44s (F), and MMP-1 (G) was performed as described in Materials and Methods.
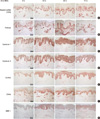
Fig. 2
Changes in HSPG mRNA expression in human male buttock skin by acute UV irradiation. Healthy young Korean male buttock skin was irradiated with 2 MED of UV and the skin specimens were obtained at 0, 24, 48, or 74 hr after UV irradiation by punch biopsy. After separation of the epidermis from the dermis, total RNA was extracted from the epidermis tissues, and the mRNA expression changes of perlecan (A), syndecan-1 (B), syndecan-4 (C), CD44 (D), agrin (E), and MMP-1 (F) by acute UV irradiation were analyzed by real-time qPCR (n = 6), as described in Materials and Methods. Data were analyzed using the comparative Ct method, normalized to 36B4, and presented as mean relative fold changes ± standard deviation. Statistical analyses were performed using a Wilcoxon signed-rank test. P values of less than 0.05 were considered statistically significant. N.D., not detected.

Fig. 3
Changes in mRNA expression levels of HS synthesizing and degrading enzymes in human male buttock skin by acute UV irradiation. Healthy young Korean male buttock skin was irradiated with 2 MED of UV and the skin specimens were obtained at 0, 24, 48, or 74 hr after UV irradiation by punch biopsy. After separation of the epidermis from the dermis, total RNA was extracted from the epidermis tissues, and the mRNA expression changes of exostosin-1 (A), exostosin-2 (B), and heparanase (C) by acute UV irradiation were analyzed by real-time qPCR (n = 6), as described in Materials and Methods. Data were analyzed using the comparative Ct method, normalized to 36B4, and presented as mean relative fold changes ± standard deviation. Statistical analyses were performed using a Wilcoxon signed-rank test. P values of less than 0.05 were considered statistically significant.
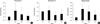
Notes
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080283), by a grant from Translational Research Center for Protein Function Control, National Science Foundation (NSF) (2009-0092961), Korea, and by a research agreement with the Amore-Pacific Corporation, Seoul, Korea.
References
1. Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: hostassociated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006. 20:9–22.
2. Oh JH, Kim YK, Jung JY, Shin JE, Chung JH. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp Dermatol. 2011. 20:454–456.
3. Oh JH, Kim YK, Jung JY, Shin JE, Kim KH, Cho KH, Eun HC, Chung JH. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011. 62:192–201.
4. Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006. 6:633–643.
5. Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006. 16:40–51.
6. Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005. 96:488–500.
7. Nedvetzki S, Golan I, Assayag N, Gonen E, Caspi D, Gladnikoff M, Yayon A, Naor D. A mutation in a CD44 variant of inflammatory cells enhances the mitogenic interaction of FGF with its receptor. J Clin Invest. 2003. 111:1211–1220.
8. Lundqvist K, Schmidtchen A. Immunohistochemical studies on proteoglycan expression in normal skin and chronic ulcers. Br J Dermatol. 2001. 144:254–259.
9. Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996. 379:335–339.
10. Chung JH, Seo JY, Choi HR, Lee MK, Youn CS, Rhie G, Cho KH, Kim KH, Park KC, Eun HC. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001. 117:1218–1224.
11. Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007. 127:687–697.
12. Werth BB, Bashir M, Chang L, Werth VP. Ultraviolet irradiation induces the accumulation of chondroitin sulfate, but not other glycosaminoglycans, in human skin. PLoS One. 2011. 6:e14830.
13. Shin JE, Oh JH, Kim YK, Jung JY, Chung JH. Transcriptional regulation of proteoglycans and glycosaminoglycan chain-synthesizing glycosyltransferases by UV irradiation in cultured human dermal fibroblasts. J Korean Med Sci. 2011. 26:417–424.
14. Kim EJ, Kim MK, Jin XJ, Oh JH, Kim JE, Chung JH. Skin aging and photoaging alter fatty acids composition, including 11,14,17-eicosatrienoic acid, in the epidermis of human skin. J Korean Med Sci. 2010. 25:980–983.
15. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003. 4:33–45.
16. Dong KK, Damaghi N, Picart SD, Markova NG, Obayashi K, Okano Y, Masaki H, Grether-Beck S, Krutmann J, Smiles KA, Yarosh DB. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp Dermatol. 2008. 17:1037–1044.
17. Iriyama S, Matsunaga Y, Takahashi K, Matsuzaki K, Kumagai N, Amano S. Activation of heparanase by ultraviolet B irradiation leads to functional loss of basement membrane at the dermal-epidermal junction in human skin. Arch Dermatol Res. 2011. 303:253–261.
18. Margelin D, Fourtanier A, Thevenin T, Medaisko C, Breton M, Picard J. Alterations of proteoglycans in ultraviolet-irradiated skin. Photochem Photobiol. 1993. 58:211–218.
19. Tzellos TG, Klagas I, Vahtsevanos K, Triaridis S, Printza A, Kyrgidis A, Karakiulakis G, Zouboulis CC, Papakonstantinou E. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009. 18:1028–1035.
20. van den Born J, Salmivirta K, Henttinen T, Ostman N, Ishimaru T, Miyaura S, Yoshida K, Salmivirta M. Novel heparan sulfate structures revealed by monoclonal antibodies. J Biol Chem. 2005. 280:20516–20523.
21. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002. 118:402–408.
22. Hašová M, Crhák T, Safránková B, Dvořaková J, Muthný T, Velebný V, Kubala L. Hyaluronan minimizes effects of UV irradiation on human keratinocytes. Arch Dermatol Res. 2011. 303:277–284.
23. Calikoglu E, Sorg O, Tran C, Grand D, Carraux P, Saurat JH, Kaya G. UVA and UVB decrease the expression of CD44 and hyaluronate in mouse epidermis, which is counteracted by topical retinoids. Photochem Photobiol. 2006. 82:1342–1347.
24. Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996. 271:10079–10086.
25. Marschall C, Lengyel E, Nobutoh T, Braungart E, Douwes K, Simon A, Magdolen V, Reuning U, Degitz K. UVB increases urokinase-type plasminogen activator receptor (uPAR) expression. J Invest Dermatol. 1999. 113:69–76.
26. Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004. 35:998–1004.
27. Liuzzo JP, Petanceska SS, Moscatelli D, Devi LA. Inflammatory mediators regulate cathepsin S in macrophages and microglia: a role in attenuating heparan sulfate interactions. Mol Med. 1999. 5:320–333.
28. Zheng Y, Lai W, Wan M, Maibach HI. Expression of cathepsins in human skin photoaging. Skin Pharmacol Physiol. 2011. 24:10–21.
29. Huh MI, Lee YM, Seo SK, Kang BS, Chang Y, Lee YS, Fini ME, Kang SS, Jung JC. Roles of MMP/TIMP in regulating matrix swelling and cell migration during chick corneal development. J Cell Biochem. 2007. 101:1222–1237.
30. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004. 204:47–54.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download