Abstract
The benefits of early enteral feeding (EEN) have been demonstrated in gastrointestinal surgery. But, the impact of EEN has not been elucidated yet. We assessed the postoperative nutritional status of patients who had undergone pancreaticoduodenectomy (PD) according to the postoperative nutritional method and compared the clinical outcomes of two methods. A prospective randomized trial was undertaken following PD. Patients were randomly divided into two groups; the EEN group received the postoperative enteral feed and the control group received the postoperative total parenteral nutrition (TPN) management. Thirty-eight patients were included in our analyses. The first day of bowel movement and time to take a normal soft diet was significantly shorter in EEN group than in TPN group. Prealbumin and transferrin were significantly reduced on post-operative day (POD) 7 and were slowly recovered until POD 90 in the TPN group than in the EEN group. EEN group rapidly recovered weight after POD 21 whereas it was gradually decreased in TPN group until POD 90. EEN after PD is associated with preservation of weight compared with TPN and impact on recovery of digestive function after PD.
Pancreaticoduodenectomy (PD) is currently considered as the treatment choice for carcinoma in periampullary regions. In recent years, this procedure has been rapidly developed and has become safer and more efficient in high volume centers (1). PD results in a loss of gastric pacemaker and a partial pancreatic resection, and such physiologic consequence leads to a high incidence of postoperative malnutrition. Though many surgeons consider that this postoperative malnutrition is an unavoidable sequence of PD, the importance of nutritional status which influences patients' quality of life, cannot be ignored.
Postoperative nutritional support was shown to reduce the incidence of complications and to shorten the hospital stay. Recently, early enteral feeding (EEN), in comparison with total parenteral nutrition (TPN), has become a standard management pathway for nutritional support after gastrointestinal surgery (2-7). However, clinical data on postoperative EEN after PD are very limited, and reports available are focused only on early postoperative results such as the safety and efficacy of EEN after PD (8-11).
In this prospective study, we assessed the postoperative nutritional status of patients who had undergone PD according to the postoperative nutritional method between EEN and TPN, and compared the clinical outcomes of the two modes.
In this open, randomized, single center, parallel group trial, we investigated a long term effect of EEN on postoperative weight change comparing with TPN management in pancreaticoduodenectomy patients. We included patients over 18 yr of age who received PD with malignant periampullary pathology at Gangnam Severance Hospital of Yonsei University Health System between May 2007 and December 2008. Exclusion criteria included: 1) a history of major abdominal or pelvic surgery; 2) patients with metastatic disease and palliative surgery; 3) a history of abdominal or pelvic radiation; 4) patients currently taking steroid or other immunosuppressive medications. Patients receiving preoperative nutritional support were also excluded.
All patients underwent exploratory laparotomy followed by pylorus preserving pancreatoduonectomy (PPPD) or conventional PD as previously described (12). Pancreaticojejunostomy was performed with duct to mucosa anastomosis in all patients. No pancreaticogastrostomies were performed. End-to-side hepaticojejunostomy was performed 15 cm proximal to the pancreaticojejunostomy with single-layer interrupted sutures. An antecolic duodenojejunostomy (or gastrojejunostomy) was constructed using a two-layered anastomosis. At the end of surgery, patients were randomized in the operating room using a sealed envelop to either EEN or TPN group. Feeding nasojejunal tube was placed into patients randomized to enteral feeding group. Just before closing the wound, 8 Fr feeding tube (Kangaroo, Sherwood Medical, Tullemore, Ireland) with the guide wire in the lumen was inserted by the anesthetist through the naris and pushed down until distal tip of the tube was 20 cm aborally from the duodenojejunostomy or gastrojejunostomy anastomosis. After reconstruction, a closed suction, silicon drain (Jackson-Pratt, Baxter Health Care Corp., Deerfield, IL, USA), was placed from the right upper quadrant posterior to the pancreaticojejunal and biliary anastomoses.
We examined nutritional status of all patients, and the parameters included weight, laboratory parameters, and the Patient Generated Subjective Global Assessment (PG-SGA) at baseline and postoperative 7th, 14th, 21th, and 90th day by a dietitian. A full diet history was performed, and energy intake and protein intake were calculated. For each component of scored PG-SGA, points (0-4) were awarded depending on the impact of the symptom on nutritional status. A score ≥ 9 indicates a critical need for nutritional intervention.
Patients were randomized into two groups, the EEN group to receive postoperative enteral feed and the control group to receive postoperative TPN management (Fig. 1). Enteral feeding (Jevity RTH, Abbott Laboratories, IL, USA) was started within 24 hr postoperatively at a rate of 20 mL/h. The velocity was progressively increased by 20 mL/d until reaching full nutritional goal (25 kcal/kg). Enteral feeding was delivered by an infusion pump for 18 h/day with 6 hr of a rest period.
In the control group, TPN was initiated on the first postoperative day. All patients received TPN solution that has 25 kcal/kg every day. The ratio of glucose to lipid in this solution was 2:1, and nonprotein calorie to nitrogen (kcal/kg) was 100:1. Multivitamins, electrolytes, trace elements and insulin were also included in the TPN solution. All nutrient solutions were prepared daily under aseptic conditions. Infusion was performed through a central venous catheter using an injection micro pump. Enteral or parenteral infusion was continued until the patient's oral intake reached approximately 800 kcal/d.
According to the policy of our department, antacid drugs for stress ulcer prophylaxis and octreotide (Sandostatin® 150 µg, Novartis, East Hanover, NJ, USA) was administered to all patients for 7 days postoperatively. Patients were given sips of water between postoperative days 4 and 5 and then proceed to a regular diet within 7 days.
Members of the surgical staff did not participate in the recorded postoperative complications. PD related complications, such as delayed gastric emptying and pancreatic fistula, were defined by the International Pancreas Study Group (13, 14). Enteral feeding related complications, abdominal cramps and distention, diarrhea (defined as more than three bowel movements per day), vomiting, and aspiration were considered adverse effects. Adverse effects were treated according to the following protocol: 1) abdominal clamping pain was treated first with analgesic drug; in patients with persistent symptoms despite the drug administration, infusion rate was reduced by 20 mL/h or temporarily stopped for 6-12 hr and resumed at a slower rate; 2) abdominal bloating was treated first by prokinetics drug; in patients with persistent symptoms despite the drug administration, infusion rate was reduced by 20 mL/h or temporarily stopped for 6-12 hr and resumed at a slower rate; 3) vomiting was treated by a temporary stop of infusion followed by diagnostic procedures; if there was no intestinal obstruction, infusion was resumed at the slower rate; 4) diarrhea was treated by reducing the infusion rate by 20 mL/h or temporarily stopping it for 6-12 hr, which was resumed at a slower rate; in patients with persistent diarrhea, Clostridium difficile infection was always ruled out.
The primary end point of the study was a change in weight. Secondary end-points were rates of delayed gastric empting and pancreatic fistula, duration of hospital stay and change of nutritional index on postoperative days 7, 14, 21, and 90. Postoperative weight loss after PD for periampullary carcinoma was 13.5%, and based on the results achieved in a previous study, the aim of this study was to reduce this weight loss rate by 50% in the group receiving the EEN (13). Based on this, a sample size of 40 (20 in each group) was necessary to show this difference at a 5% significance with a power of 80%, allowing for a drop out rate of 10%.
Continuous variables are expressed as mean ± standard deviation (SD). Differences in variables between groups were tested using Student's t-test, chi-square test, or Fisher's exact test. However, nonparametric tests (Kruskal-Wallis or Mann-Whitney test) were used for variables with skewed distributions. P values less than 0.05 were considered statistically significant.
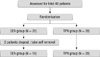
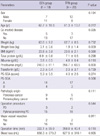
A total of 40 patients were recruited and randomized to either the TPN or EEN treatment group (Fig. 2). Two patients withdrew from the study because their nasojejunal tubes were accidentally dislodged. Thirty-eight patients (18 EEN and 20 TPN) were analyzed. The mean age of patients was 61.0 yr (± 11.9 yr) and consisted of 19 men and 19 women. Five patients underwent PD, and 33 underwent PPPD. Pathologic diagnoses were pancreatic carcinoma in 14 patients, bile duct cancer in 11, adenocarcinoma of the ampulla of Vater in 11, and duodenal carcinoma in 3. The patient demographics and nutritional parameters of both groups are shown in Table 1. The preoperative weight loss significantly changed in the EEN group compared to the TPN group. The two groups showed no significant differences in age, sex, comorbidity, operative blood loss and preoperative nutritional index.
The first day of bowel movement and the time to take a soft diet was significantly shorter in EEN group than in TPN group. Hospital stay was shorter in the EEN group without significance (Table 2). There were no cases of hospital mortality. Details of complications are shown in Table 2. Overall, 13 of 38 patients (34.2%) had postoperative complications, and the complication rates of two groups were similar to each other. Pancreatic fistula and delayed gastric emptying were the most common complications in this study.
Overall, 3 of 38 patients developed pancreatic fistula as defined by the ISGPF criteria (13) and the overall incidence was 7.9%. Two (66.7%) patients had grade A, and the other (33.3%) grade C. Pancreatic fistula grade C occurred in the EEN group and was resolved after reinsertion of percutaneous drainage.
Overall, 3 of 38 patients developed delayed gastric emptying (DGE) as defined by the ISGPS criteria (14) and the overall incidence was 7.9%. Two (66.7%) patients had grade A, and one (33.3%) grade C. Chyle abdomen developed in one patient in each group, which was resolved after temporary reduction of enteral nutrition and oral feeding.
There were no aspiration episodes or enteral feeding associated intestinal ischemia. Enteral nutrition was relatively well tolerable to the patients. In EEN group, 4 of 18 patients developed side effects for enteral nutrition. For instance, one patient had diarrhea, one had an abdominal distention, one had a sore throat, and one had nausea and vomiting. All side effects were relieved with a conservative management and a temporary reduction of the amount of enteral nutrition.
All nutritional parameters decreased until POD 7 and increased gradually thereafter.
The level of serum albumin, and total protein decreased, and PG-SGA in the early postoperative days and gradually increased in the late postoperative days, but there was no significant difference between two groups (Table 3). In the EEN group, body weight gradually decreased until POD 14, but rapidly recovered on POD 21. In contrast, body weight gradually decreased until POD 90 in TPN group (P = 0.005). The rapid turnover proteins such as prealbumin and transferrin were more significantly reduced on POD 7 and slowly recovered until POD 90 in the TPN group than in the EEN group (Fig. 3).
Traditionally, feeding for patients after gastrointestinal surgery started when flatus or defecation indicated the return of bowel function. However, in recent years, early enteral nutrition in gastrointestinal surgery should be recommended whenever possible. The benefits of EEN have been demonstrated to be more physiological, better preventive in morphologic and functional alteration of the gut system, and less expensive than TPN (15-17).
Pancreatic leakage is one of the leading postoperative complications after PD, and it can lead to prolonged hospital stay, increased costs and mortality. Consequently, pancreatic surgeons often prefer postoperative TPN because of the greater risk of pancreatic leakage. However, several reports have suggested that there is no marked difference in pancreatic leakage between EEN and TPN groups (11, 18). Our study is consistent with previous reports that EEN is not a significant factor for pancreatic leakage.
In contrast to the report of Martignoni et al. (11), there were no significant differences in the occurrence of delayed gastric emptying between the EEN and TPN groups. The main difference between the report of Martignoni et al and the present study was the cyclic infusion of enteral nutrition. Certainly, the cyclic enteral nutrition has advantages for patients over continuous enteral nutrition because it is closer to the natural form of enteral nutrition (19). Our results demonstrate that EEN does not increase the incidence of delayed gastric emptying after PD. Moreover, EEN promotes faster recovery of bowel peristalsis by reducing time to recanalize for passing gas and feces.
Our results are not consistent with the findings of Brennan et al. (9), who suggested that TPN was associated with increased infectious complications. The main difference between the report of Brennan et al. and this study was caloric load. They provided high calories at a rate of 30-35 kcal/day, which is considered relative overfeeding today, and this overfeeding may increase postoperative complications when compared with permissive underfeeding (20). However, a routine use of TPN does not seem to provide any benefit because the high rate of glucose intolerance observed in TPN patients.
There are several methods to deliver enteral nutrition after PD. Nasojejunal tube provides a cost effective and desirable method of enteral nutrition without the morbidity from an additional enterotomy (21). However, the frequency of nasojejunal tube dislodgement and occlusion was as high as 35% to 100% (22, 23). In the present study, nasojejunal feeding tube was used for all EEN patients, and jejunostomy tube was not used according to our department policy. That is because any unnecessary enterotomy was a potential source of complications. There were no catheter related major complications in the EEN group except for accidentally dislodged nasojejunal tube. In our experience, tube occlusion can be prevented by irrigating it with 20 mL water every 8 hr and after giving medications. The accidentally dislodgement of nasojejunal tube was the most common phenomenon, and special education and attention must be paid to firm nasal fixation. We think that a nasojejunal tube is an effective tool for providing enteral feeding after PD.
In our study, EEN related adverse effect occurred in 22.2% and were resolved by reduction or temporary interruption of infusion. All patients in EEN group reached the nutritional goal without having difficulty by following the infusion protocol. This may be attributed to the fact that our protocol started at a slow flow rate with careful and progressive increase in the feeding volume. Also, this can prevent abdominal distension caused by reaching the nutritional goal too aggressively and early (24).
In usual practice, the parenteral nutrition begins at goal rates, whereas the enteral feedings advance to goal rate over several days. Although the level of rapid turnover proteins such as prealbumin and transferrin dropped in all the patients after the operation, it was recovered significantly fast in the EEN group in the early postoperative period. These results indicate that EEN modulates a metabolic response, favoring the synthesis of proteins.
It has been shown that patients can maintain a normal body weight after surgery, but it is frequently less than their preoperative body weight (25-27). Kozuscheck et al. (28) reported that 85% of patients who had undergone PPPD reached the preoperative body weight one year after surgery. However, in the present study, preoperative body weight restored in 3 weeks in the EEN group, and recovery of weight 3 weeks after the operation was significantly better in the EEN patients than in the TPN patients. The restoring of the preoperative body weight depends on a many factors including appetite. The mechanisms involved are unclear, but most probably it reflects that EEN stimulate the appetite, so the faster normalization of the dietary intake would be the reason for the significantly faster achievement of the preoperative weight in the EEN group. This finding is important, considering the growing number of elderly patients undergoing PD for periampullary carcinoma.
The use of objective nutrition parameters (anthropometric and biochemical) to assess nutritional status has been questioned in the view of the many non-nutritional factors affecting the results. The scored PG-SGA has been accepted by the Oncology Nutrition Dietetic Practice Group of the American Dietetic Association as the standard for nutrition assessment for cancer patients. The present cut-off score of 9 is appropriate for the initiation of urgent nutritional intervention. In the present study, The PG-SGA score was the highest on POD 14, although other biochemical nutritional indexes were within a normal range on that day. So, we recommend the PG-SGA score should be screened for patients after PD and it may prevent or delay deterioration in the patient's nutritional status by providing early nutrition support.
The present study has some limitations. The first, weakness is that patients who had undergone conventional PD were included in this study. Indeed, the operation procedures could attribute to postoperative nutritional status. Some studies have shown that PPPD was associated with better nutritional status postoperatively as compared to conventional PD (18, 29, 30). However, we believed that there were no significant differences in the nutritional status because two procedures are equally allocated in the two groups. Second, it is possible that nutritional outcomes of EEN after PD might have been underestimated in this study because of the small sample size study. Therefore, trials with large sample size are required to demonstrate whether postoperative and long-term quality of life is better and whether nutritional outcomes are improved.
In conclusion, postoperative EEN is safe and well tolerated, and shows no negative effect on anastomosis healing. Furthermore, EEN improves early and long term postoperative nutritional status and whole body protein kinetics. It is recommended that the routine postoperative enteral feeding for patients undergoing PD is beneficial and should be a standard of care.
Figures and Tables
Fig. 3
Mean prealbumin and transferring levels on preoperative day and on days 7, 14, 21, and 90 postoperatively*. Error bars: 95% confidence interval. *There was significant difference between the EEN and TPN Group at any time point of postoperative days.
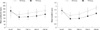
References
1. Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000. 232:786–795.
2. Yi JM, Hur H, Kim SK, Song KY, Chin HM, Kim W, Park CH, Park SM, Lim KW, Jeon HM. Is a fast-tract critical pathway possible in gastric cancer surgery? J Korean Gastric Cancer Assoc. 2009. 9:18–25.
3. DiFronzo LA, Yamin N, Patel K, O'Connell TX. Benefits of early feeding and early hospital discharge in elderly patients undergoing open colon resection. J Am Coll Surg. 2003. 197:747–752.
4. Farreras N, Artigas V, Cardona D, Rius X, Trias M, González JA. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr. 2005. 24:55–65.
5. Feo CV, Romanini B, Sortini D, Ragazzi R, Zamboni P, Pansini GC, Liboni A. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004. 74:298–301.
6. Jolliet P, Pichard C, Biolo G, Chioléro R, Grimble G, Leverve X, Nitenberg G, Novak I, Planas M, Preiser JC, Roth E, Schols AM, Wernerman J. Working Group on Nutrition and Metabolism, ESICM. European Society of Intensive Care Medicine. Enteral nutrition in intensive care patients: a practical approach. Intensive Care Med. 1998. 24:848–859.
7. Soop M, Carlson GL, Hopkinson J, Clarke S, Thorell A, Nygren J, Ljungqvist O. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg. 2004. 91:1138–1145.
8. Baradi H, Walsh RM, Henderson JM, Vogt D, Popovich M. Postoperative jejunal feeding and outcome of pancreaticoduodenectomy. J Gastrointest Surg. 2004. 8:428–433.
9. Brennan MF, Pisters PW, Posner M, Quesada O, Shike M. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 1994. 220:436–441.
10. Gianotti L, Braga M, Gentilini O, Balzano G, Zerbi A, Di Carlo V. Artificial nutrition after pancreaticoduodenectomy. Pancreas. 2000. 21:344–351.
11. Martignoni ME, Friess H, Sell F, Ricken L, Shrikhande S, Kulli C, Büchler MW. Enteral nutrition prolongs delayed gastric emptying in patients after Whipple resection. Am J Surg. 2000. 180:18–23.
12. Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, Lee WJ, Chi HS. Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery. 2009. 146:882–887.
13. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005. 138:8–13.
14. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007. 142:761–768.
15. Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001. 29:242–248.
16. Gianotti L, Alexander JW, Nelson JL, Fukushima R, Pyles T, Chalk CL. Role of early enteral feeding and acute starvation on postburn bacterial translocation and host defense: prospective, randomized trials. Crit Care Med. 1994. 22:265–272.
17. Johnson CD, Kudsk KA. Nutrition and intestinal mucosal immunity. Clin Nutr. 1999. 18:337–344.
18. Di Carlo V, Gianotti L, Balzano G, Zerbi A, Braga M. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg. 1999. 16:320–326.
19. van Berge Henegouwen MI, Akkermans LM, van Gulik TM, Masclee AA, Moojen TM, Obertop H, Gouma DJ. Prospective, randomized trial on the effect of cyclic versus continuous enteral nutrition on postoperative gastric function after pylorus-preserving pancreatoduodenectomy. Ann Surg. 1997. 226:677–685.
20. Zaloga GP, Roberts P. Permissive underfeeding. New Horiz. 1994. 2:257–263.
21. Dark DS, Pingleton SK, Kerby GR. Hypercapnia during weaning. A complication of nutritional support. Chest. 1985. 88:141–143.
22. Keohane PP, Attrill H, Jones BJ, Silk DB. Limitations and drawbacks of 'fine bore' nasogastric feeding tubes. Clin Nutr. 1983. 2:85–86.
23. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987. 11:187–189.
24. Watters JM, Kirkpatrick SM, Norris SB, Shamji FM, Wells GA. Immediate postoperative enteral feeding results in impaired respiratory mechanics and decreased mobility. Ann Surg. 1997. 226:369–377.
25. McLeod RS, Taylor BR, O'Connor BI, Greenberg GR, Jeejeebhoy KN, Royall D, Langer B. Quality of life, nutritional status, and gastrointestinal hormone profile following the Whipple procedure. Am J Surg. 1995. 169:179–185.
26. Melvin WS, Buekers KS, Muscarella P, Johnson JA, Schirmer WJ, Ellison EC. Outcome analysis of long-term survivors following pancreaticoduodenectomy. J Gastrointest Surg. 1998. 2:72–78.
27. van Berge Henegouwen MI, Moojen TM, van Gulik TM, Rauws EA, Obertop H, Gouma DJ. Postoperative weight gain after standard Whipple's procedure versus pylorus-preserving pancreatoduodenectomy: the influence of tumour status. Br J Surg. 1998. 85:922–926.
28. Kozuschek W, Reith HB, Waleczek H, Haarmann W, Edelmann M, Sonntag D. A comparison of long term results of the standard Whipple procedure and the pylorus preserving pancreatoduodenectomy. J Am Coll Surg. 1994. 178:443–453.
29. Klinkenbijl JH, van der Schelling GP, Hop WC, van Pel R, Bruining HA, Jeekel J. The advantages of pylorus-preserving pancreatoduodenectomy in malignant disease of the pancreas and periampullary region. Ann Surg. 1992. 216:142–145.
30. Wenger FA, Jacobi CA, Haubold K, Zieren HU, Müller JM. Gastrointestinal quality of life after duodenopancreatectomy in pancreatic carcinoma. Preliminary results of a prospective randomized study: pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy. Chirurg. 1999. 70:1454–1459.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download