Abstract
Recent studies reported that early initiation of hemodialysis may increase mortality. However, studies that assessed the influence of early initiation of peritoneal dialysis (PD) yielded controversial results. In the present study, we evaluated the prognosis of early initiation of PD on the various outcomes of end stage renal failure patients by using propensity-score matching methods. Incident PD patients (n = 491) who started PD at SNU Hospital were enrolled. The patients were divided into 'early starters (n = 244)' and 'late starters (n = 247)' on the basis of the estimated glomerular filtration rate (eGFR) at the start of dialysis. The calculated propensity-score was used for one-to-one matching. After propensity-score-based matching (n = 136, for each group), no significant differences were observed in terms of all-cause mortality (P = 0.17), technique failure (P = 0.62), cardiovascular event (P = 0.96) and composite event (P = 0.86) between the early and late starters. Stratification analysis in the propensity-score quartiles (n = 491) exhibited no trend toward better or poorer survival in terms of all-cause mortality. In conclusion, early commencement of PD does not reduce the mortality risk and other outcomes. Although the recent guidelines suggest that initiation of dialysis at higher eGFR, physicians should not determine the time to initiate PD therapy simply rely on the eGFR alone.
In 1997, the National Kidney Foundation workgroup recommended that dialysis should be initiated when the creatinine (Cr) clearance falls below approximately 10.5 mL/min/1.73 m2 except in well-nourished, asymptomatic patients (1). In 2006, however, the NKF workgroup updated the guidelines for initiating dialysis at the estimated glomerular filtration rate (eGFR) < 15.0 mL/min/1.73 m2. They also suggested that initiation of dialysis even at eGFR > 15.0 mL/min/1.73 m2 may be appropriate in patients who have symptoms related to both their comorbidities and their level of residual renal function (RRF) (2). The Canadian Society of Nephrology recommends the initiation of dialysis when the eGFR is less than 12 mL/min/1.73 m2 and the Caring for Australians with Renal Impairment Guidelines specify eGFR of 10.0 mL/min/1.73 m2 as the standard on which nephrologists should rely for starting dialysis therapy if there is evidence of uremia or its complications such as malnutrition (3, 4). These guidelines have influenced the clinical practice of nephrologists. According to the United States Renal Data System, an increase in the frequency of patients initiating dialysis with eGFR above 10 mL/min/1.73 m2 is becoming evident (5).
Recent observational studies have not shown any benefits of early start of dialysis and some studies reported that early initiation of dialysis may increase mortality (6-10). The Initiating Dialysis Early and Late (IDEAL) study, the first randomized controlled trial, demonstrated that early initiation of dialysis in patients with chronic kidney disease stage 5 was not associated with an improvement in clinical outcomes (11). However, the IDEAL study could not address any differences between peritoneal dialysis (PD) or hemodialysis in terms of the association of the start eGFR and the subsequent outcomes, since the above study comprised similar proportion of PD and hemodialysis patients.
If dialysis modality was concerned, survival disadvantage of early start of dialysis would be a different story. Studies that solely assessed patients treated with hemodialysis indicated a higher mortality in the early initiation group (6, 7, 12). In contrast, studies that assessed PD patients showed controversial results. Shiao et al. (13) demonstrated that early initiation of PD had significant survival disadvantage. However, Tang et al. (14) showed increased mortality risk with decreased start eGFR at initiation of PD. Therefore, it is not determined whether early initiation of PD is beneficial or detrimental for the patient outcomes. Compared with hemodialysis, PD has a beneficial effect in terms of preserving residual renal function (RRF) (15, 16), a major determinant of various outcomes after commencing dialysis therapy (17, 18). Therefore, it is warranted to investigate the effect of early initiation of PD treatment on the patient survival and other outcomes.
The present study explored the benefit of the early initiation of PD in overall survival and other outcomes retrospectively. Propensity score (PS) analysis was employed to account for the differences of pre-dialysis factors and one-to-one matching between early and late starter groups.
We conducted the present study with retrospective data collection using PD patient database of Seoul National University Hospital (SNUH). All patients with chronic kidney disease are given unbiased information on the choice of dialysis modality at SNUH. The choice for dialysis modality is made at the discretion of the patients themselves, although they receive medical advice from the nephrologists and dialysis nurses. On the average, 20 to 25% of end stage renal failure (ESRF) patients choose PD as their first-line dialysis therapy in SNUH. Patients with impending ESRF who accepted PD as their primary modality were subjected to Tenckhoff catheter insertion, usually followed by a break-in period of two weeks for tunnel healing and step-wise training for exchange procedure. They started PD after the break-in period. Patients who developed uremic symptoms or uremic emergency during this period were temporarily given hemodialysis therapy until the start of PD. A total of 593 patients commenced PD at SNUH from January, 2000 until July, 2010. Among them, we excluded patients who had previously received hemodialysis for > 1 month or started dialysis because of a failing renal allograft. Patients were also excluded if they died or switched to hemodialysis within 3 months after starting PD or discontinued PD due to recovering renal function. Excluded patients were not significantly different from the included patients with respect to age, sex ratio, body mass index (BMI), and comorbidity. Finally, 491 PD patients were selected and retrospectively analyzed in the present study. Demographic, clinical and outcomes data were retrieved from the PD registry data in SNUH PD Center. These data included age, sex, underlying renal disease, laboratory test results, comorbidity, vintage, and physician in charge. Laboratory tests such as highly sensitivie C-reactive protein (hsCRP), hemoglobin (Hb), albumin at the time of commencing PD were employed for analysis. Degree of comorbidity was assessed based on the Davies comorbidity score, which comprises seven comorbid conditions, leading to three risk groups, i.e., low (no comorbid disease), intermediate (1 or 2 comorbid diseases) and high (3 or more comorbid diseases) risk groups (19).
Patients with eGFR < 30 mL/min/1.73 m2 are regularly followed by a nephrologist at 4- to 6-week interval. For them, eGFR was estimated by abbreviated Modification of Diet in Renal Disease (MDRD) equation. PD patients were divided into 'early starters (n = 244)' and 'late starters (n = 247)' on the basis of the eGFR measured within 6 weeks before start of dialysis, using median value of 7.7 mL/min/1.73 m2.
The choice of particular PD system such as brand and manual vs automated PD was left to the discretion of the patients. Dialysis regimens, i.e., the number of exchanges and choice of PD solutions were adjusted by nephrologists to meet the Kt/V target of > 1.7 and achieve euvolemic status. Patients were regularly monitored for dialysis adequacy, peritoneal solute transport rate, peritonitis, and exit site infection.
All-cause mortality was the primary outcome of the present study. Data regarding conversion to hemodialysis therapy, kidney transplantation, transfer to another center, and loss to follow-up were censored in the all-cause mortality analysis. Deaths within three months of transfer to hemodialysis were deemed to be PD-related mortalities. Technical failure, cardiovascular (CV) event, and composite event defined as either CV event or patient death were analyzed, as well. Technical failure was defined as transfer to hemodialysis therapy because of peritonitis, ultrafiltration failure, inadequate dialysis, exit-site and/or tunnel infection, and mechanical problems. CV event was defined as new development of myocardial infarction, angina, fatal or nonfatal stroke, or peripheral vascular disease. Coronary angioplasty, coronary artery bypass graft, carotid endarterectomy, or peripheral revascularization procedure were also included in the definition of CV event.
SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Normality of data distribution was tested using the Kolmogorov-Smirnov test. Variables without a normal distribution were either transformed into logarithmic scale and subjected to parametric tests or analyzed by nonparametric test. Values with normal distribution are expressed as mean standard deviation, while those without normal distribution were shown as median and interquartile range. For continuous variables, comparison between two groups was made by using Student t test or Mann Whitney U test. Chi square test or Fisher's exact test was used for categorical variables.
The early starter group differed markedly from late starter group with respect to the observed pre-dialysis covariates. Therefore, we decided to account for such differences between early and late starter groups by developing a PS for being an early starter group. PS analysis is a post hoc statistical method that estimates the impact of a treatment when subjects are not randomly assigned to a specific treatment group. It supports to simultaneously control all the known patient factors that might be related with the time of PD commencement. Logistic regression was performed to calculate the risk, or the propensity of each patient to be included in the early starter group. Hosmer-Lemeshow's test was performed to evaluate the goodness-of-fit of our model. The calculated PS was either used for one-to-one matching in estimating the benefit of early initiation of PD in multivariate analysis or for stratified analysis of the outcome by the quartiles of the PS. Comparisons between PS matched pairs were performed by using paired t-test for continuous variables and McNemar test for categorical variables.
Kaplan-Meier analysis was employed to determine all-cause mortality, technical failure, and CV event free survival. In an analysis for the hazard ratio (HR) for the primary and secondary outcomes, the models were checked for proportionality assumptions. Then, a multivariate Cox proportional hazards model with the aid of forward selection was used to adjust the covariates for the effect of early dialysis initiation and to estimate the HR and associated 95% confidence interval (CI), since the assumptions of a constant HR over time were met. At all the statistical analysis, P < 0.05 was considered statistically significant.
The study population was incident PD patients who underwent Tenckhoff catheter insertion and subsequently started PD at SNUH from January 2000 to July 2010. Among 593 patients who commenced PD during that period, 491 PD patients were selected and analyzed by calculating the PS to be in the early starter group in the present study.
For the overall population, the median length of follow-up was 25 months, 5-yr patient survival rate was 84.3%, and 5-yr technique survival was 74.5%. The study population was classified into early and late starter groups on the basis of median GFR (7.7 mL/min/1.73 m2) at the time of commencing PD. The baseline characteristics of the two groups are detailed in the Table 1. Estimated GFR for early and late starter groups were 10.8 ± 2.5 and 5.5 ± 1.3 mL/min/1.73 m2, respectively (P < 0.001). Early starters exhibited older age, male preponderance, higher comorbidity scores including diabetes, congestive heart failure, ischemic heart disease, peripheral artery diseases, compared to the late starter group.
The variables included in the logistic regression model for the calculation of the PS were age, sex, BMI, albumin, hsCRP, diabetes, congestive heart failure, malignancy, ischemic heart disease, peripheral vascular disease, connective tissue disease, school education years, commencing year, and physician (Hosmer-Lemeshow's test, P = 0.6311).
One-to-one propensity matching between the two groups was performed according to the PS. The baseline characteristics of the PS-matched pairs (n = 136) is described in the Table 1. No difference was observed with respect to the age, gender ratio, BMI, Hb, albumin, hsCRP, comorbidty parameters and education years.
Post hoc analysis was performed by defining quartiles of the PS and estimating the outcomes in each quartile by Kaplan-Meier method. Here, we selected to employ stratification in quartiles, instead of quintiles, because of the limited size of our patient population.
One-to-one PS matching provided 136 pairs of patients who underwent early or late initiation of PD (Table 1). During the median follow-up of 27 months, 14 of 136 patients in early starter group and 10 of 136 patients in the late starter group died (Table 2 and 3, adjusted hazard ratio with early initiation 0.47, 95% confidence interval [CI] 0.16 to 1.35, P = 0.17). As shown in the Fig. 1, no significant differences were observed in terms of all-cause mortality (P = 0.17), technique failure (P = 0.62), CV event (P = 0.96) and composite event (P = 0.86) between the early and late starters.
Stratification analysis in the PS quartiles (n = 491, Table 4) showed no trend toward better or poorer survival in terms of all-cause mortality.
The present study showed that the all cause of mortality, technical failure, CV event, and composite event were not different between early and late starters of peritoneal dialysis therapy when we estimated outcomes with PS matched pairs model. In a stratification analysis by the PS quartiles, all-cause mortality was not different across the quartiles of PS.
It had been suggested that early initiation of dialysis might improve nutrition and quality of life. It had also been suggested that patients initiated dialysis early had less uremic complication with consequent decrease in hospitalization, mortality, and cost (20, 21). The evidence for these was that lower GFR at the initiation of dialysis was associated with lower serum albumin level, which, in turn, correlated with higher mortality (22). Therefore, it had been suggested that early initiation of dialysis will prevent malnutrition and thereby decrease mortality. Several observational cohort and case-control studies so far also showed that early initiation of dialysis was associated with improved survival rate (23, 24). However, these studies did not account for lead-time bias or comorbidities.
In contrast, other observational studies and a recent randomized controlled trial have suggested that initiation of dialysis at higher eGFR had no effect on survival or worsened survival rate (5-12, 25, 26). However, serum Cr depends not only on the RRF but also on the muscle mass. Lower serum Cr at dialysis commencement might indicate both better RRF and poorer nutrition with lower muscle mass. Such lower muscle mass could partly account for the apparent association between start eGFR and subsequent mortality risk (27).
The recently published randomized controlled trial, the IDEAL study, found no survival benefit of early dialysis initiation (11). This study randomly allocated the 828 ESRF patients of Australia and New Zealand to early or late starter groups and followed them up for 3.59 yr. However, the mean eGFR difference between two groups of IDEAL study was only 1.8 mL/min/1.73 m2 (early starters; 9.0 mL/min/1.73 m2 vs late starters; 7.2 mL/min/1.73 m2). Besides, the study participants comprised similar proportions of hemodialysis and PD patients and did not address the effect of start eGFR on the survival with respect to the PD alone. RRF is better preserved with PD than hemodialysis (14, 15) and the RRF is an important prognostic factor in ESRF patients (16, 17). Therefore, results from the IDEAL study could not be directly translated into PD.
In our retrospective analysis for the effect of early vs late PD commencement on the various outcomes, we employed two types of models using PS in order to eliminate the bias due to comorbidities. In a retrospective study, initiation of dialysis depends more heavily on the grade of comorbidity rather than eGFR, since uremic symptom is more closely associated with the comorbidity than eGFR. In the present study, factors that generally lead to a poor prognosis, such as old age, lower albumin, higher hsCRP, diabetes, congestive heart failure, ischemic heart disease, and peripheral vascular disease, were more common in early starters than in late starters before matching. Therefore, by employing PS matching method, we tried to eliminate the possible effect of comorbid conditions. After matching, all the pre-dialysis covariates became similar between the two groups, only except eGFR at dialysis initiation (10.4 2.2 vs 5.8 1.2 mL/min/1.73 m2, P < 0.001). In contrast to the IDEAL study, the present study exhibited a remarkable difference of eGFR between the two groups.
The present study showed 14 out of 136 from early starter group and 10 out of 136 late starter group died from any cause during median 27-months follow-up. This indicates a lower mortality rate, compared with other reports from the Caucacians (28). Better clinical outcomes of Asian PD patients, as compared to the western PD population, are also reported elsewhere (29, 30).
The present study has several potential limitations. First, although as many pre-dialysis covariates as available were included in the PS calculation, other determinants might remain unrecognized in this retrospective study. Second, MDRD equation was used to calculate eGFR at initiation timing of PD. As mentioned above, lower serum Cr could be a feature of both a better RRF and a poorer nutritional status. Although our PS calculation included the albumin level, detailed information for other nutritional parameters was absent. Lastly, lead time bias might have an influence at early stages of follow-up period and this effect might disappear at later stages.
In conclusion, early commencement of PD does not reduce the mortality risk and other outcomes in our study. Although the recent guidelines suggest that initiation of dialysis at higher eGFR, physicians should not determine the time to initiate PD therapy simply rely on the eGFR alone. PD may be delayed until traditional clinical indications for dialysis are present.
Figures and Tables
Fig. 1
Primary and secondary outcomes of early and late starters of peritoneal dialysis adjusted by start age, sex, body mass index, albumin, hemoglobin, hsCRP, diabetes, congestive heart failure, malignancy, peripheral artery disease, ischemic heart disease, collagen disorder, and education level. (A) All-cause mortality (P = 0.17). (B) Technique failure (P = 0.62). (C) Cardiovascular event (P = 0.96). (D) Composite event (P = 0.86) free survival.

Table 3
Multivariate Cox regression analysis for overall mortality
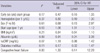
*Adjusted for start age, sex, BMI, albumin, Hb, hsCRP, diabetes, congestive heart failure, malignancy, peripheral artery disease, ischemic heart disease, collagen disorder, and education level. HR, hazard ratio; CI, confidence interval; BMI, body mass index; hsCRP, highly sensitive C-reactive protein.
ACKNOWLEDGMENTS
We are grateful for the statistical support from the Medical Research Collaborating Center (MRCC), Seoul National University Hospital, Seoul National University College of Medicine.
References
1. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 1997. 30:S67–S136.
2. Peritoneal Dialysis Adequacy Work Group. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis. 2006. 48:S98–S129.
3. Churchill DN, Blake PG, Jindal KK, Toffelmire EB, Goldstein MB. Clinical practice guidelines for initiation of dialysis. J Am Soc Nephrol. 1999. 10:S289–S291.
4. Kelly J, Stanley M, Harris D. Caring for Australians with Renal Impairment (CARI). The CARI guidelines: acceptance into dialysis guidelines. Nephrology (Carlton). 2005. 10:S46–S60.
5. Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009. 76:257–261.
6. Kazmi WH, Gilbertson DT, Obrador GT, Guo HF, Pereira BJ, Collins AJ, Kausz AT. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005. 46:887–896.
7. Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011. 171:396–403.
8. Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010. 5:1828–1835.
9. Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010. 77:700–707.
10. Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009. 24:3175–3182.
11. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA. IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010. 363:609–619.
12. Wilson B, Harwood L, Locking-Cusolito H, Chen SJ, Heidenheim P, Craik D, Clark WF. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007. 11:263–269.
13. Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Perit Dial Int. 2008. 28:73–81.
14. Tang SC, Ho YW, Tang AW, Cheng YY, Chiu FH, Lo WK, Lai KN. for the Hong Kong Peritoneal Dialysis Study Group. Delaying initiation of dialysis till symptomatic uraemia: is it too late? Nephrol Dial Transplant. 2007. 22:1926–1932.
15. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006. 69:1726–1732.
16. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000. 11:556–564.
17. Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S. Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002. 13:1307–1320.
18. Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010. 56:348–358.
19. Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002. 17:1085–1092.
20. Hakim RM, Lazarus JM. Initiation of dialysis. J Am Soc Nephrol. 1995. 6:1319–1328.
21. Churchill DN. An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis. 1997. 30:899–906.
22. Kopple JD, Greene T, Chumlea WC, Hollinger D, Maroni BJ, Merrill D, Scherch LK, Schulman G, Wang SR, Zimmer GS. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD Study. Kidney Int. 2000. 57:1688–1703.
23. Kim SG, Kim NH. The effect of residual renal function at the initiation of dialysis on patient survival. Korean J Intern Med. 2009. 24:55–62.
24. Liu H, Peng Y, Liu F, Xiao H, Chen X, Huang A, Liu Y. Renal function and serum albumin at the start of dialysis in 514 Chinese ESRD in-patients. Ren Fail. 2008. 30:685–690.
25. Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Taiwan Society of Nephrology. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010. 25:2616–2624.
26. Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009. 24:3186–3192.
27. Szczech LA, Reddan DN, Klassen PS, Coladonato J, Chua B, Lowrie EG, Lazarus JM, Owen WF. Interactions between dialysis-related volume exposures, nutritional surrogates and mortality among ESRD patients. Nephrol Dial Transplant. 2003. 18:1585–1591.
28. Stel VS, van de Luijtgaarden MW, Wanner C, Jager KJ. on behalf of the European Renal Registry Investigators. The 2008 ERA-EDTA Registry Annual Report: a précis. NDT Plus. 2011. 4:1–13.
29. Lee JE, Oh KH, Choi KH, Kim SB, Kim YS, Do JY, Kim YL, Kim DJ. Statin therapy is associated with improved survival in incident peritoneal dialysis patients: propensity-matched comparison. Nephrol Dial Transplant. 2011. 26:4090–4094.
30. Han SH, Lee SC, Ahn SV, Lee JE, Choi HY, Kim BS, Kang SW, Choi KH, Han DS, Lee HY. Improving outcome of CAPD: twenty-five years' experience in a single Korean center. Perit Dial Int. 2007. 27:432–440.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download