Abstract
A recent resurgence of pertussis has raised public health concerns even in developed countries with high vaccination coverage. The aim of this study was to describe the clinical characteristics of infant pertussis, and to determine the relative importance of household transmission in Korea. The multicenter study was prospectively conducted from January 2009 to September 2011. We identified the demographic and clinical data from these patients and performed the diagnostic tests for pertussis in their household contacts. Twenty-one patients with confirmed pertussis were included in the analysis. All infections occurred in infants younger than 6 months of age (mean age, 2.5 months) who had not completed the primary DTaP vaccination except for one patient. Infants without immunization history had a significant higher lymphocytosis and longer duration of hospital stay compared to those with immunization. All were diagnosed with PCR (100%), however, culture tests showed the lowest sensitivity (42.9%). Presumed source of infection in household contacts was documented in 85.7%, mainly parents (52.6%). Pertussis had a major morbidity in young infants who were not fully immunized. Household members were responsible for pertussis transmission of infants in whom a source could be identified. The control of pertussis through booster vaccination with Tdap in family who is taking care of young infants is necessary in Korea.
Pertussis, also known as whooping cough, is an acute respiratory tract infection caused by Bordetella pertussis. Although the incidence rate of pertussis has significantly decreased following the wide use of pertussis vaccine, B. pertussis infection is continuously occurring as a small outbreak even in several developed countries with high vaccination coverage (1-4). There has been the resurgence of reported pertussis cases primarily in infants younger than 1 yr old, especially less than 3 months of age, in adolescents and young adults worldwide (5, 6). The possible causes of increased incidence in adolescents and adults include waning of vaccine immunity, adaptation of circulating B. pertussis strains, development of diagnostic methods, and active surveillance due to increased awareness of pertussis (7-9). Household contact with infected adolescents and adults also becomes the major source of pertussis infection in infants who are not fully immunized, and this problematic circulation may consequently threaten overall public health (10, 11).
The epidemiological characteristics of pertussis can vary depending on the definition of case, diagnostic method of confirmed case, national reporting system, network organization for epidemiological investigation, and the local vaccination schedule. National immunization program (NIP) of Korea consists of three primary series of diphtheria-tetanus-acellular pertussis vaccine (DTaP) at 2, 4, and 6 months, followed by a first booster at 15-18 months and a second booster between 4 and 6 yr of age. Recently, a tetanus toxoid, reduced diphtheria and acellular pertussis (Tdap) booster vaccine in adolescents aged 11-12 yr is added to Korean NIP in 2012. The vaccination rate of primary DTaP continues to be approximately 94% (12, 13), and there is a mandatory notification system in Korea. An annual average of about 11.5 cases of pertussis has been reported to the Korea Center for Disease Control and Prevention (KCDC), however, the reported cases of pertussis have increased since the 2000s. In addition, the KCDC reported that the incidence of pertussis was markedly increased (more than about 5.5 times) in 2009, compared to the previous (14). Also, the incidence of pertussis in 2011 was more than two times compared with the incidence in 2009 (15). We may predict the substantial outbreak in the future in our country.
Given the importance of accurately determining the epidemiological features of infant pertussis and the lack of reliable existing data in Korea, this study was conducted to describe the clinical characteristics of laboratory confirmed cases less than 1 yr of age and to evaluate the relative importance of family members on infants who are vulnerable to B. pertussis transmission.
A prospective, multicenter, observational study was conducted between January 2009 and September 2011. Three hospitals participated in the study: Seoul St. Mary's hospital; Suwon St. Vincent's hospital; and Incheon St. Mary's hospital. We evaluated all infants clinically suspected of pertussis infection because of a cough lasting at least 2 weeks with at least one of the following symptoms: paroxysmal coughing; inspiratory whooping; post-tussive vomiting or apnea without other known cause. We obtained information on current respiratory manifestations, radiologic findings and immunization status for each infant. Diagnostic approaches for pertussis were conducted for clinical cases. Nasopharyngeal aspirates (NPA), or swab samples if aspirates were not possible, and blood samples were collected within 2 days at admission. Laboratory tests were performed at the Vaccine Bio Institute (VBI) of The Catholic University of Korea. It is determined as the criteria for laboratory-confirmed pertussis case when the subjects is applicable to one of the following criteria: 1) positive result of B. pertussis on culture of nasopharyngeal aspirates (NPA) or swab; this sample was collected and cultured on Regan-Lowe culture medium at 37℃ for more than 1 week; 2) positive result of B. pertussis in PCR or real-time PCR (RT-PCR) of NPA or swab; PCR was done by the method Glare et al. reported (16), and RT-PCR was done by modified method of Reischl U. Colleagues manual (17); 3) positive serology which defined as pertussis toxin (PT) antibody in a single serum sample that was higher than cut-off value (24 EU/mL) of enzyme-linked immunosorbent assay (ELISA) kit (IBL, Hamburg, Germany) or a 4-fold increased change in anti-PT antibody between acute-phase and convalescent-phase serum.
After the laboratory confirmation on pertussis, the parent or legal guardian for registration of index case was contacted as soon as possible, and all infants who are eligible for inclusion criteria were registered to the study immediately upon receipt of the consent form. To all family members if cough started at least 7 days before the onset of symptoms in the index case, it was requested to participate in the study as household contacts. And they were registered for the study immediately upon receipt of the consent form, and interviewed using the standard questionnaire to collect demographic and clinical data. Also, for all long-term household contacts, it was asked to visit for the study to collect respiratory samples (for culture, PCR, RT-PCR identification of B. pertussis) and/or serum samples (for ELISA).
Student's t-test and Fisher's exact test were applied for comparing categorical variables between the groups. Data were analyzed using SPSS statistical software, version 15.0 for Windows (Chicago, IL, USA). P values less than 0.05 were considered statistically significant throughout analysis.
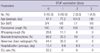
Of a total of 65 clinically suspected cases, 21 infants (32.3%) were enrolled to be the confirmed cases of B. pertussis infection in this study. The age range was from 22 to 198 days old with a mean age of 2.5 months old. There were 11 males and 10 females. All were born full term at birth. Nine patients (42.9%, average age: 47.4 days) had not received any DTaP vaccinations, 9 (42.9%, average age: 77.2 days) received 1 dose, 2 (9.5%, average age: 104.5 days) received 2 doses, and 1 (4.8%, age: 198 days) received 3 doses of DTaP (Table 1). Pertussis occurred mostly in spring, summer and early fall, peaking in April, June, and September. There was no patient in the winter month from December to February (Fig. 1).
Although paroxysmal cough manifested for more than 1 week in all cases, whooping cough was present in only 3 cases (14.3%). There were 4 cases (19.0%) of pneumonic infiltration, 3 cases (14.3%) of hyperinflation on radiologic examinations. Four patients with abnormal chest radiographic finding were not immunized with DTaP vaccination, and remaining 3 patients were received with 1 dose of DTaP vaccination. All patients except 2 cases were examined by multiplex PCR for respiratory virus identification. Two patients had concomitant infections of rhinovirus and respiratory syncytial virus, respectively, and severity was not increased in these patients. Absolute lymphocytosis was noted in 12 patients (57.1%). Of note, absolute lymphocytosis was found more commonly in patients (8/9, 88.9%, average age: 45.8 days) who did not receive DTaP vaccination than patients (4/12, 33.3%, average age: 88.0 days) who did receive DTaP vaccination (P = 0.024, Table 2). The mean length of hospital stay among all patients was 11.7 days. However, the mean length of hospitalization for patients without DTaP vaccination was 15.4 ± 6.6 days, which was significantly longer than the 8.8 ± 3.8 days of patients with DTaP vaccination (P = 0.009, Table 2). Two patients were admitted to the intensive care unit (ICU); both of them did not receive DTaP vaccination, and one of these patients treated with a mechanical ventilator. There were no deaths due to pertussis during the study period (Table 1).
On the result of diagnostic methods, PCR had the highest sensitivity showing positivity for all 21 cases (100%), however, confirmed cases with culture were 9 (42.9%) which showed the lowest sensitivity. Although RT-PCR and serological test were not performed in all patients, high sensitivity was observed in all patients done with these tests.
Total of 72 family members and long-term guardians of index infant cases were enrolled in this study for confirming household transmission. The composition of those members were as followings; 42 parents, 20 siblings, 10 relatives. Seventy members (97.2%) showed respiratory symptoms, and 42 members (60%) with respiratory symptoms had a history of antibiotics use. Of these 72 family members, 38 members (38/72, 52.8%) were confirmed with laboratory criteria of pertussis as followings; all 38 members were positive by PCR, 31 members (who received ELISA assay) were all positive serology, 12 members were positive culture. Finally, a total of 18 potential family sources (18/21, 85.7%) were identified for confirmed cases, of which 20 members (20/38, 52.6%, 15 family) were parents and 8 (8/38, 21.1%, 6 family) were siblings. Other 10 members (6 family; 6 grandparents, 4 aunts) accounted for 26.3% (10/38). In particular, mothers provided the highest proportion (13/38, 34.2%) of presumed sources of infection (Fig. 2).
A resurgence of reported pertussis has been documented in a number of countries with high vaccine coverage since the 1990s (1, 4, 18). DTaP vaccination coverage in Korea is very high, with 94% of the children completing the primary series of pertussis immunization. Despite the nationwide vaccination program with high coverage, the incidence of reported pertussis has continued to increase from 2000. Understanding the epidemiological and clinical features of pertussis in community is important because it remains a major cause of morbidity and mortality worldwide. In this study, we have made the demographic data from confirmed pertussis in an attempt to compare the clinical outcomes according to DTaP immunization. Generally, pertussis mostly occurred in young infants under 6 months of age who did not complete the primary series of DTaP vaccination. Depending on the immunization status of our patients, there were significant differences in clinical outcome similar to other studies (11, 19, 20). The duration of hospital stay was significantly longer; and the severe cases admitted to ICU were found in patients without pertussis vaccination as compared to those with vaccination. The absolute lymphocytosis in laboratory findings was shown primarily in patients without the vaccination history. The main presenting symptom in typical pertussis is whooping cough. Even though all patients had paroxysmal cough for more than 1 week, whooping cough was present in only 14.3% of the patients. In addition, most patients showed no specific findings in chest radiographs. Because the presenting symptoms may be nonspecific, it is important for clinicians to suspect pertussis when there is prolonged cough or absolute lymphocytosis, especially in young infants with no or incomplete DTaP vaccination.
The highest numbers of pertussis cases was in April, June, and September, which have relatively warm temperatures in Korea. Few patients occurred in winter as other respiratory pathogens are commonly considered in the differential diagnosis. Similar studies from other countries have also shown that the reported pertussis case mostly occurred in a summer months with high temperatures (21, 22). Since the number of patients is too small, it is unclear to know the seasonality of pertussis based only upon this result. In addition, various factors such as environmental factors, school opening, and climate can affect the seasonal characteristics of pertussis epidemiology. However, because clinical presentations of pertussis often resemble influenza-like symptoms and those of other respiratory diseases such as Mycoplasma pneumoniae and adenovirus, this lack of awareness and nonspecific clinical characteristics may be often associated with underdiagnosed condition of pertussis.
The diagnostic tool of pertussis is challenging. Culture of nasopharyngeal secretions is essential for diagnosis, however, the result showed the lowest sensitivity (42.9%) in our patients. Moreover, this result requires a long incubation period up to 10 to 14 days, after which the patient can be delayed in critical treatment and infect other persons with contact. The majority of confirmed cases were based in the presence of PCR. Although RT-PCR and serological test were not performed in all patients, these methods also have higher sensitivity than culture. Serologic testing can help the diagnosis of patients with atypical symptoms, for whom clinical samples are collected at later time from the onset of disease.
This study is, to our knowledge, the first to document the presumed importance of household transmission in Korea. In 18 families (85.7%) of our patients where a source of infection was established, parents (52.6%), grandparents and aunts (26.3%), or siblings (21.1%) were identified as the frequent source. In particular, mothers provided the highest proportions of presumed sources of infection.
In our study, the majority of household members showed respiratory symptoms such as prolonged cough and recent history of antibiotics treatment for respiratory infections. However, none of them did know that pertussis might cause severe or chronic cough in adolescents and adults. Also, they did not have the concept of household transmission of pertussis. There has been an increase in the reported number of pertussiss cases in many countries, primarily among adolescents and adults, which is special concern because adolescents and young adults are a recognized reservoir of infection for neonates and infants, who are at high risk for pertussis-related morbidity and mortality (23-26). Several household studies have noted the predominant role of parents in the transmission of pertussis to susceptible infants (27, 28). For this reason, the USA Advisory Committee on Immunization Practices (ACIP) has recommended the cocooning strategy that Tdap vaccine be administered to all caregivers of infants less than 1 yr, in an effort to protect young infants against pertussis since 2006 (29). Likewise, the cocooning strategy is important in Korea, in keeping with high morbidity and mortality in young infants without immunization (30). Despite the introduction of Tdap vaccine in our country, vaccine use is only beginning, perhaps because of an incomplete understanding of the burden of pertussis among community population and the lack of national policy and education concerned with the necessity of Tdap vaccination. The immunization policy in Korea recently introduced the booster dose of Tdap vaccine as part of routine immunization in adolescent. Therefore, the change in the epidemiology of pertussis should be noted carefully.
In conclusion, pertussis is still present in Korea and remains a major morbidity in young infants. Most infants were too young to have received the primary series of DTaP vaccination before infection. Household members were identified as a potential source of pertussis infection, and they should be encouraged to receive booster immunization with Tdap to minimize pertussis transmission to this vulnerable group on infants. Nationwide pertussis reporting is also urgently required to better understand disease transmission patterns, for early recognition and prevention of outbreak and for the evaluation of vaccination policy. Universal recommendation for pertussis booster vaccination at 11 to 12 yr is expected to decrease the transmission of pertussis in households in Korea. The surveillance of pertussis outbreak should be continued for controlling this disease.
Figures and Tables
References
1. Crespo I, Cardenosa N, Godoy P, Carmona G, Sala MR, Barrabeig I, Alvarez J, Minguel S, Camps N, Cayla J, et al. Epidemiology of pertussis in a country with high vaccination coverage. Vaccine. 2011. 29:4244–4248.
2. Güriş D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, Wharton M, Livengood JR. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin Infect Dis. 1999. 28:1230–1237.
3. Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE. Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005. 24:761–765.
4. Wymann MN, Richard JL, Vidondo B, Heininger U. Prospective pertussis surveillance in Switzerland, 1991-2006. Vaccine. 2011. 29:2058–2065.
5. Edwards KM. Overview of pertussis: focus on epidemiology, sources of infection, and long term protection after infant vaccination. Pediatr Infect Dis J. 2005. 24:S104–S108.
6. Crowcroft NS, Pebody RG. Recent developments in pertussis. Lancet. 2006. 367:1926–1936.
7. Cherry JD. The science and fiction of the "resurgence" of pertussis. Pediatrics. 2003. 112:405–406.
8. Wood N, McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008. 9:201–211. quiz 11-2.
9. Mooi FR, van Loo IH, van Gent M, He Q, Bart MJ, Heuvelman KJ, de Greeff SC, Diavatopoulos D, Teunis P, Nagelkerke N, et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg Infect Dis. 2009. 15:1206–1213.
10. Greenberg DP, von König CH, Heininger U. Health burden of pertussis in infants and children. Pediatr Infect Dis J. 2005. 24:S39–S43.
11. Elliott E, McIntyre P, Ridley G, Morris A, Massie J, McEniery J, Knight G. National study of infants hospitalized with pertussis in the acellular vaccine era. Pediatr Infect Dis J. 2004. 23:246–252.
12. Kim EY, Lee MS. Related factors of age-appropriate immunization among urban-rural children aged 24-35 months in a 2005 population-based survey in Nonsan, Korea. Yonsei Med J. 2011. 52:104–112.
13. Park B, Lee YK, Cho LY, Go UY, Yang JJ, Ma SH, Choi BY, Lee MS, Lee JS, Choi EH, et al. Estimation of nationwide vaccination coverage and comparison of interview and telephone survey methodology for estimating vaccination status. J Korean Med Sci. 2011. 26:711–719.
14. Hong JY. Update on pertussis and pertussis immunization. Korean J Pediatr. 2010. 53:629–633.
15. CDC. Surveillance data of nationally notifiable communiable diseases (annual reporting pertussis). accessed on 12 August 2012. Available at http://www.cdc.go.kr.
16. Glare EM, Paton JC, Premier RR, Lawrence AJ, Nisbet IT. Analysis of a repetitive DNA sequence from Bordetella pertussis and its application to the diagnosis of pertussis using the polymerase chain reaction. J Clin Microbiol. 1990. 28:1982–1987.
17. Reischl U, Lehn N, Sanden GN, Loeffelholz MJ. Real-time PCR assay targeting IS481 of Bordetella pertussis and molecular basis for detecting Bordetella holmesii. J Clin Microbiol. 2001. 39:1963–1966.
18. Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics. 2011. 3:183–188.
19. Mikelova LK, Halperin SA, Scheifele D, Smith B, Ford-Jones E, Vaudry W, Jadavji T, Law B, Moore D. Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr. 2003. 143:576–581.
20. Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009. 28:194–198.
21. Lin YC, Yao SM, Yan JJ, Chen YY, Chiang CS, Wu HS, Li SY. Epidemiological shift in the prevalence of pertussis in Taiwan: implications for pertussis vaccination. J Med Microbiol. 2007. 56:533–537.
22. Skowronski DM, De Serres G, MacDonald D, Wu W, Shaw C, Macnabb J, Champagne S, Patrick DM, Halperin SA. The changing age and seasonal profile of pertussis in Canada. J Infect Dis. 2002. 185:1448–1453.
23. Wirsing von König CH, Postels-Multani S, Bogaerts H, Bock HL, Laukamp S, Kiederle S, Schmitt HJ. Factors influencing the spread of pertussis in households. Eur J Pediatr. 1998. 157:391–394.
24. Baron S, Njamkepo E, Grimprel E, Begue P, Desenclos JC, Drucker J, Guiso N. Epidemiology of pertussis in French hospitals in 1993 and 1994: thirty years after a routine use of vaccination. Pediatr Infect Dis J. 1998. 17:412–418.
25. Goh A, Chong CY, Tee N, Loo LH, Yeo JG, Chan YH. Pertussis--an under-diagnosed disease with high morbidity in Singapore children. Vaccine. 2011. 29:2503–2507.
26. Simmerman JM, Chu D, Chang H. Implications of unrecognized severe acute respiratory syndrome. Nurse Pract. 2003. 28:21–31.
27. Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, Rebmann CA, Gabel J, Schauer SL, Lett SM. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004. 23:985–989.
28. Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007. 26:293–299.
29. Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006. 55:1–37.
30. Nilsson L, Lepp T, von Segebaden K, Hallander H, Gustafsson L. Pertussis vaccination in infancy lowers the incidence of pertussis disease and the rate of hospitalisation after one and two doses: analyses of 10 years of pertussis surveillance. Vaccine. 2012. 30:3239–3247.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download