Abstract
Cervical cancer is the second most common gynecological cancer among Korean women. While nationwide screening program has developed, the pathogenesis of cervical cancer is unknown. The aim of this study was to compare the protein expression profiles between cervical squamous carcinomas and normal cervical tissues in order to identify proteins that are related to the cancer. Three cervical cancer tissue samples and three normal cervical tissue samples were obtained and protein expression was compared and was identified in the samples with the use of matrix assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). A total of 20 proteins that showed up-regulated expression in the cervical cancer tissue samples were selected and identified. Seven proteins were matched to allograft inflammatory factor 1 (AIF-1), actine-like protein 2 (ALP2), brain type fatty acid-binding protein (B-FABP), NCK adaptor protein 1 (NCK-1), islet cell autoantigen 1 (ICA69), cationic trypsinogen (PRSS1), and cyclin-dependent kinase 4 (CDK4), but the remaining 13 proteins were unidentifiable. After confirmation by RT-PCR, Western blotting and immunohistochemistry, we found that B-FABP, NCK-1, and CDK4 were related to the pathogenesis of cervical cancer. These proteins are suggested as candidates of new pathological tumor markers for cervical cancer.
Cervical cancer is the second most common gynecological cancer among Korean women and accounts for 9.8% of new female cancer cases (1). A total of 44,182 patients diagnosed with cervical cancer between 1993 and 2002 have been reported to the Korea Central Cancer Registry and the Gynecologic Oncology Committee of the Korean Society of Obstetrics and Gynecology. The incidence rate of an adenocarcinoma has been reported as 1.2% in 1993-1995 and 1.4% in 1999-2002, while the incidence rate for a squamous cell carcinoma (SCC) declined from 15.1% in 1993-1995 to 12.2% in 1999-2002 (2). There is strong evidence that has suggested that cervical SCC is 100% attributable to infection with certain types of human papillomavirus (HPV); and the World Health Organization (WHO) has recently recognized that this cancer is caused by HPV (3, 4).
Most cells contain thousands of proteins and some over-expressed proteins in cancer cells can be released into the blood-stream, where the levels of the proteins can be measured. For serological tumor markers of cervical cancers, the sensitivity for cytokeratin fragment 21.1 (CYFRA 21.1), carcinoembryonic antigen (CEA) and SCC antigen have been reported as 26%, 25%, and 43%, respectively at diagnosis. SCC is considered as the tumor marker of choice for SCC and the addition of CEA or CYFRA 21.1 does not significantly increase the sensitivity obtained by the determination of the level of SCC alone (5). Even though the level of SCC antigen is still considered as the most accurate measurement for cervical carcinoma, determination of the SCC level is not applicable for the screening of early cervical cancer. Early cervical cancer has been mostly detected by the use of the Papanicolaou test with the highest accuracy. However, most cervical cancers are caused by persistent high-risk HPV (HR-HPV) infection. Most efforts have focused on the detection of HR-HPV related antibodies. There is evidence that the detection of anti-L1 antibodies could be successfully used for the discrimination between patients with cervical lesions and women with a normal cytology (6). Such phenomena may be detected and classified by the evaluation of differential protein or gene expression in cancer tissues. Although HPV infection is a recognized cause of cervical cancer, HPV infection alone is not sufficient for carcinogenesis (7).
Proteomics may also have a critical role in the rapid identification of new protein targets and help to elucidate the underlying molecular events associated with the development and progression of cancer. The aim of this study was to compare the protein expression profiles between cervical cancer tissues and normal cervical tissues in order to identify proteins related to the cancer. Expression profiles were determined with the use of the proteomic analysis of two-dimensional electrophoresis and matrix-assisted laser desorption and ionization time of flight mass spectrometry (MALDI-TOF-MS). Candidate proteins were also identified by the use of RT-PCR, Western blotting, and immunohistochemical staining. These candidate proteins may provide useful information regarding early detection of cervical SCC.
Between January and June, 2010, cervical cancer tissues were obtained from 3 patients who had radical hysterectomy in Korea University Hospital and normal cervical tissues from 3 patients who had simple hysterectomy due to myomas as non-cancerous controls.
All samples were stored at -70℃ and histopathological diagnosis was initially performed. The cancer tissues and normal cervical tissues were subsequently used for proteomic analyses.
Each sample was mixed and homogenized with a sample buffer (5 M urea, 2 M thiourea, 2 mM TBP, 2% CHAPS, 2% sulfobetadine, 0.5% carrier ampholytes, 40 mM Tris, protease inhibitor in a total volume of 0.5 mL with water). After ultracentrifugation, supernatants of each samples were collected. The protein concentration of each sample was quantified by use of the Bradford method with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). After five minutes of incubation at room temperature, the absorbance of each sample was measured at 595 nm and the protein quantity was calculated.
Isoelectric focusing (IEF) instrumentation, immobilized pH gradient gel (IPG) strips (17 cm, pH 3-10 or 17 cm, pH 4-7), and related reagents were purchased from Bio-Rad. IPG strips were rehydrated, and were focused for an automated overnight procedure in ceramic strip holders, using an 8-hr rehydration period, followed by 1 hr each at 500, 1,000, and 2,000 V, respectively, followed by 5,000 V, for a total of 50 kVh. Immediately prior to the loading of the focused IPG strips on the two-dimensional gels, the strips were incubated in equilibrium buffer (6 M urea, 10% sodium dodecylsulfate [SDS], pH 8.8 Tris, glycerol, dithiothreitol [DTT]) for 15 min, followed by a 15-min incubation in the same solution, except that DTT was replaced by iodoacetamide. Two-dimensional SDS-PAGE 12% gels (acrylamide/bis 30% T, 2.67% C, distilled water, 1.5 M Tris-HCl pH 8.8, 10% SDS, 10% ammonium persulfate, 0.1% tetra-methyl-ethylenediamine [TEMED]) were then cast with the use of glass plate sandwiches from Bio-Rad Protean II xi chambers (20 × 20 cm, 1.5 mm thickness). The SDS-equilibrated IPG gels were sealed on top of the two-dimensional gels using 0.5% agarose with the addition of bromophenol blue. SDS gels were then electrophoresed until the tracking dye was within 1 cm of the bottom of the gel.
Silver staining as described by Orsmark et al. (8) was done to visualize the proteins, and the gel images were scanned. The scanned two-dimensional (2D) gels were then analyzed using the Progenesis workstation (version 2003.03, Nonlinear Dynamics, Durham, NC, USA) image analysis software. After standardization and warping of all the protein spots using image analysis, unique spots as well as protein spots that exhibited a three-fold or higher increase in the normalized volume as compared to the normal counterpart images, were selected as significant protein spots for further analysis. Three invasive cervical cancer gels were compared with three normal cervical tissue gels as controls and protein spots that had corresponded to proteins where expression was up-regulated or down-regulated in the invasive cervical carcinoma samples by more than three-fold according to densitometry were then used for protein analysis.
The selected protein spots were subjected to in-gel trypsin digestion and analysis using MALDI-TOF-MS. The stained protein spots of interest were excised and small pieces of the gels were destained with 20 mM potassium ferricyanide and 100 mM sodium thiosulfate. The protein spots were then washed in distilled water. After a reaction with 150 µL of 200 mM ammonium bicarbonate for 20 min, the proteins were again washed in distilled water. The protein samples were dehydrated repeatedly with acetonitrile and were dried in a speed vacuum for 30 min. After being allowed to react with 30 µL of 50 mM ammonium bicarbonate and 5 µL of trypsin (0.1 µg/µL), the protein samples were reactivated for 12-16 hr at 37℃, and were then incubated with 100 µL 50 mM ammonium bicarbonate solution in a shaker for 1 hr at 37℃. After centrifugation, each supernatant was dehydrated with 100 µL acetonitrile for 10 min at 37℃. Each supernatant was collected after the repeated dehydration process was then dried for more than 6 hr. MALDI-TOF analysis was ultimately performed on these dried samples.
Alpha-cyano-1-hydroxycinnamic acid (Sigma, St. Louis, MO, USA) was used as a matrix. One microliter of the matrix (10 mg/mL) and 1 µL of the eluted peptides were deposited on a MALDI plate for MALDI-TOF-MS analysis. The tryptic peptides were separated and analyzed by MALDI-TOF-MS in order to obtain mass fingerprints of the peptide fragments from the proteins that were separated on the 2D gels. MALDI-TOF-MS was conducted using a Voyager-DE-STR TOF-MS (Applied Biosystems, Foster City, CA, USA) equipped with a model VSL-337ND nitrogen laser (Spectra Physics, Mountain View, CA, USA; 337 nm, 3 ns pulse length) and a dual microchannel plate detector (Galileo Electro-Optics, Sturbridge, MA, USA). The acceleration voltage in the ion source was 20,000 V. Reflector mode and delayed extraction conditions were used in this study. Protein identification was based on comparison of the peptide masses of the MALDI-TOF spectra experimentally derived from the isolated proteins by enzymatic digestion with corresponding theoretical peptide masses calculated for all the protein sequences present in the NCBI protein database. This comparison was conducted using Sequest (Sequest, Lisle, IL, USA) software.
RT-PCR was performed to confirm the expression of up-regulated proteins in the cervical cancer samples. Total RNA was extracted from frozen samples (two cervical cancer tissues, two normal cervical tissues) using TRIZOL reagent (Life Technologies, Gaithersburg, MD, USA). A volume of 200 µL of chloroform (Sigma) was added to each sample for 10 min, and centrifugation progressed for 15 min at 4℃. The supernatants from each sample were isolated and 500 µL of isopropanol was added. After 10 min incubation at room temperature and 10 min centrifugation at 4℃, the isopropanol was removed and 1 mL of 75% cold ethanol was added. Centrifugation was conducted for an additional 8 min at 4℃, and the cold ethanol was removed. After drying for 5 min at room temperature, the pellet was melted with 50 µL of DEPC.
After preparation, 2 µg of total RNA was added to 0.5 µg of oligo(dT)12-18 or random hexamer (Life Technologies) for 10 min at 70℃. Total RNA per reaction was reverse-transcribed into the cDNA using Moloney murine leukemia virus reverse transcriptase and 1 × buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM DTT) for 1 hr at 42℃. Denaturation was performed for 5 min at 95℃ in order to halt the activity of the reverse transcriptase after the reaction. The primer sequences and product sizes are listed in Table 1.
The PCR cycle progressed as follows: 94℃ for 30 sec, 54-64℃ for 30 sec and 72℃ for 1 min 30 sec. This protocol was repeated for 30 cycles (for β2-microglobulin) or 35 cycles (for other genes) and a final extension step for 4 min at 72℃ was performed. A control PCR reaction containing Taq DNA polymerase and primer combination, but with no template, was used as a negative PCR control. The PCR products were then separated on a 1.5% agarose gel and were visualized with the use of ethidium bromide staining.
For Western blotting, two cervical cancer tissue and two normal cervical tissue samples were used. Tissue samples were placed in a micro-tissue grinder that contained lysis buffer, and were centrifuged at 12,000 × g for 5 min. Protein (50 µg) was collected from the supernatant fraction and was electrophoresed on a 7.5% SDS-polyacrylamide gel (SDS-PAGE); and the separated proteins were electrophoretically transferred to a Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) for 2 hr at 0.8 mA/cm2 at 4℃.
After blocking with 5% nonfat dry milk in phosphate buffered saline (PBS) for 2 hr at room temperature, filters were incubated on a shaker with goat polyclonal anti-NCK1, anti-B-FABP or CDK-4 (1:500) in PBS for 1 hr at room temperature. Binding of the primary antibody was detected by chemiluminescence with a mouse peroxidase-conjugated secondary antibody IgG (Vectastain ABC kit, Vector Lab, Burlingame, CA, USA) at 1:500 in PBS-milk for 90 min, visualized with ECL Western blotting detection reagents (Amersham Pharmacia Biotech) and exposed to ECL film (Amersham Pharmacia Biotech).
Paraffin embedded cervical cancer and normal cervical tissue samples were sectioned at 5 µm, immersed in methanol containing 0.3% H2O2 for 20 min and then digested with 2 ng/mL hyaluronidase in 0.1 M acetate buffer, pH 5.2 for 30 min at room temperature, and were then washed (3 × 5 min). Nonspecific sites were blocked with 1.5% normal donkey serum in PBS for 1 hr. Sections were incubated overnight at 4℃ in a humidified chamber with goat polyclonal anti-NCK1 or anti-B-FABP or CDK-4 at a dilution of 1:50-1:200 (2 µg/mL) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequent steps were all carried out at room temperature. Sections were washed three times in PBS and were treated with an avidin-biotin complex (goat ABC staining system, Santa Cruz Biotechnology) as a secondary antibody, and peroxidase-conjugated avidin as a chromogen after allowing 1 hr for primary and secondary antibody conjugation. Diaminobenzidine (DAB) was used as a chromogen and hematoxylin was used as a counter-stain. Slides were observed at 100 ×, 200 ×, and 400 × magnification using an Olympus light microscope (Vanox-S type; Olympus, Tokyo, Japan).
More than 2,000 protein spots in both cervical cancers and normal cervical tissues were identified with the use of 2D electrophoresis. After filtering and editing the images, 1,400 spots were detected in the cervical cancer samples and 1,250 spots were identified in the normal cervical tissues, within a size range of 24-116 kDa and a pH range of 4-7. The protein spots that exhibited unique expression and a three-fold or higher increase in normalized volume as compared to counterpart images were selected as significant protein spots. As the result of a comparative analysis of the spots between both the normal and cancer specimens, about 200 proteins were determined as having up-regulated expression in the cervical cancer tissues. Among these proteins, 20 proteins that were also identified as having up-regulated expression by more than three-fold in density than in the corresponding normal cervical tissues were selected (Fig. 1). These proteins were determined as acidic proteins that ranged in molecular weight from 24 to 95 kDa over a pH-region of 4.8 to 6.7. This expression pattern was reproducible in each cancer sample.
Among the 20 proteins with up-regulated expression in the cervical cancer samples, seven proteins were matched to the allograft inflammatory factor 1 (AIF-1), actin-like protein 2 (ALP2), brain type fatty acid-binding protein (B-FABP), NCK adaptor protein 1 (NCK-1), islet cell autoantigen 1 (ICA69), cationic trypsinogen (PRSS1), and cyclin-dependent kinase 4 (CDK4) (Fig. 1, Table 1). Thirteen of these proteins could not be matched, even after the PCR amplification of possible genes (data not shown).
RT-PCR was performed for AIF-1, ALP2, B-FABP, NCK-1, ICA69, PRSS1, and CDK4. Expression levels for these seven genes were much higher in the cervical cancer tissues than in the normal cervical tissues at the mRNA level (Fig. 2, Table 2). The protein β2-microglobulin was evenly expressed between both the normal and cervical cancer samples.
Western blotting showed the presence of distinct protein bands on autoradiographs that corresponded to NCK1, B-FABP, and CDK-4, which were observed at approximately 15 kDa, from cervical cancer samples; however, the expression levels of these proteins were low in normal cervical tissues (Fig. 3).
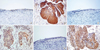
Immunostaining for B-FABP, NCK-1, and CDK4 was not observed in normal cervical tissues, but positive staining was observed in the cervical cancer tissues (Fig. 4). B-FABP, NCK-1, and CDK4 immunoreactivity was seen in the cytoplasm, cytoplasm and nucleus of cells, respectively. All three cancer samples and three normal controls showed reproducible expression patterns of B-FABP, NCK-1, and CDK4 in RT-PCR, Western blotting and immunohistochemistry.
By the use of two-dimensional electrophoresis and silver staining, we compared the protein expression profile of normal cervical tissue and cervical cancer tissue and could identify 200 up-regulated proteins. Of the proteins with up-regulated expression, 20 proteins were selected and were identified by MALDI-TOF. Although 13 proteins were not matched, seven proteins including AIF-1, ALP2, B-FABP, NCK-1, ICA69, PRSS1, and CDK4 were identified in this study.
AIF-1 is a cytoplasmic, calcium-binding, inflammation-responsive scaffold protein that has been implicated in the regulation of inflammation and the AIF-1 gene located on chromosome 6p21.3, which is densely clustered with genes involved in the inflammatory response, including surface glycoproteins, complement cascade, TNFα, TNFβ, and NF-κB genes (9). It has been reported that AIF-1 is closely associated with cardiac allograft vasculopathy, rheumatoid arthritis, inflammatory skin disorders and systemic sclerosis (8, 10). AIF-1 can promote the growth of breast tumors via the activation of NF-κB signaling, which consequently up-regulates the expression of cyclin D1 (11). In this study, expression of AIF-1 was up regulated in cervical cancer tissues, which may be the result of inflammation and abnormal proliferation in the cervical epithelial tissues.
ALP2, also named actin-related protein gene, is located on chromosome 1p36.32 and is a member of a family of actin-related proteins that share significant amino acid sequence identify to conventional actions. Among these proteins, ARP2/3 complex, which is regulated by the Wiskott-Aldrich syndrome protein (WASP) and is a suppressor of the c-AMP-receptor (Scar, also known as WAVE) has been reported to be associated with cell migration and invasion by initiating lamelliopodia and filopodia (12). Overexpression of ARP2/3 has been reported in the colon, lung, and breast cancer (13-15). However, in gastric cancer the role of ARP2/3 is controversial (16). A correlation between actin-related protein and cervical carcinogenesis has not been established but in one report, in contrast to our findings, expression of ARP3 was found to be down regulated in cervical cancer by proteomic analysis and a further investigation is needed to clarify the function of ARP in carcinogenesis of cervical cancer (17).
B-FABP is one of a family of FABPs that are members of a superfamily of intercellular lipid-binding proteins which have roles in transport and storage of lipids. B-FABP has been reported to be expressed in radial glial cells of the developing central nervous system and in malignant glioma cell lines (18). Recent studies have demonstrated gene expression profiling of B-FABP in various cancers including renal cell carcinoma, prostate cancer, and hepatocellular carcinoma (19-21). In renal cell carcinoma, B-FABP has been detected in urine and it has been suggested that B-FABP can be used as a marker for diagnosis of renal cell carcinoma (22).
NCK-1 has been recognized to mediate signaling pathways that link membrane receptors to cytoskeleton rearrangement and modulate eIF2α phosphorylation by the action of dsRNA-activated protein kinase receptor (PKR), where the main function of PKR is to mediate interferon antiviral host response (23). NCK-1 prevents efficient activation of PKR by dsRNA. In a HR-HPV target, PKR is involved in a pathway with multiple mechanisms where the level of phosphorylated PKR is decreased through a post-transcriptional mechanism mediated by E6 and E7 that is independent of the transcriptional downregulation mediated by HPV (24). Our results strongly suggest that up-regulation of NCK-1 is correlated with HPV induced carcinogenesis of cervical cancer.
ICA69 has been reported as a polypeptide antigen expressed in pancreatic beta cells, and autoimmunity against this antigen has been shown to be associated with insulin-dependent diabetes mellitus. ICA 69 has been reported to be released by pancreatic cancer cells (25).
PRSS1, located on chromosome 7q35, has been shown to have a strong association with hereditary pancreatitis and hereditary pancreatic cancer (26). Further evaluation is required to elucidate PRSS1 up-regulation expression in cervical cancer tissue.
CDK4, located on chromosome 12q14, is a protein-serine kinase involved in cell proliferation during G1 phase and is inhibited by p16. A high concentration of a cyclin D1 and CDK4 complex can result in the functional inactivation of retinoblastoma protein (pRb) and this pathway is commonly targeted in various malignancies (27). In cervical cancer, binding of HPV E7 protein to Rb leads to release of E2F transcription factor omitting the role of cyclin D1, and CDK4 has been shown a stepwise increase from normal to tumor tissues occurs, indicating an important role at an early stage of transformation of HPV infected cervical epithelium (28, 29). In this study, expression of CDK4 was up regulated in cervical cancer tissue and this finding suggests that CDK4 is closely correlated with carcinogenesis of cervical cancer.
In summary, proteomic analysis has identified approximately 200 proteins with up-regulated expression, of which 20 proteins were selected and were identified by the use of MALDI-TOF. Seven proteins were matched to AIF-1, ALP2, B-FABP, NCK-1, ICA69, PRSS1, and CDK4. After confirmation by RT-PCR, Western blotting and immunohistochemistry, we found that B-FABP, NCK-1, and CDK4 were related to the pathogenesis of cervical cancer. These proteins are suggested as candidates of new pathological tumor markers for cervical cancer. More studies to confirm the usefulness of these candidate proteins in clinical field should be warranted.
Figures and Tables
Fig. 1
Comparison of 2D PAGE proteins separated from cervical cancer tissue and normal cervical tissue samples. A total of 20 over-expressed protein spots in cervical cancer tissues were analyzed by MALDI-TOF-MS. Of the 20 proteins, seven proteins were matched to AIF-1, ALP2, B-FABP, NCK-1, ICA69, PRSS1, and CDK4 and 13 of these proteins could not be matched, even after PCR amplification of possible genes. MW, molecular weight; AIF-1, allograft inflammatory factor 1; ALP2, actin-like protein 2; B-FABP, brain type fatty acid binding protein; NCK-1, NCK adaptor protein 1; ICA69, islet cell autoantigen; PRSS1, cationic trypsinogen; CDK4, cyclin-dependent kinase 4.
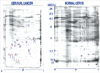
Fig. 2
RT-PCR demonstrates that the expression levels of AIF-1, ALP2, B-FABP, NCK-1, ICA69, PRSS1, and CDK4 are higher in cervical cancer tissues than in normal cervical tissues. β2-microglobulin is evenly expressed in both normal and cervical cancer tissues. AIF-1, allograft inflammatory factor 1; ALP2, actin-like protein 2; B-FABP, brain type fatty acid binding protein; NCK-1, NCK adaptor protein 1; ICA69, islet cell autoantigen; PRSS1, cationic trypsinogen; CDK4, cyclin-dependent kinase 4; L, ladder (100 bp); cervical cancers (C1, C2); normal cervical tissues (N1, N2).
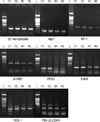
Fig. 3
Western blotting shows distinct protein bands on autoradiographs corresponding to NCK-1, B-FABP, and CDK-4, which are observed at approximately 15 kDa, 15 kDa, and 15 kDa in cervical squamous cell carcinoma (C1, C2), but the expression levels are low in normal cervical tissues (N1, N2). NCK-1, NCK adaptor protein 1; B-FABP, brain type fatty acid binding protein; CDK4, cyclin-dependent kinase 4.

Fig. 4
Immunostaining of NCK-1, B-FABP, and CDK4 are not observed in normal cervical tissues, but positive staining is observed in cervical cancer tissues. (A, C) and (E) normal cervical tissues with negative staining of NCK-1, B-FABP, and CDK. (B, D) and (F) cervical cancer tissues with positive staining of NCK-1, B-FABP, and CDK. B-FABP, brain type fatty acid binding protein; NCK-1, NCK adaptor protein 1; CDK4, cyclin-dependent kinase 4.
References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: Incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Chung HH, Jang MJ, Jung KW, Won YJ, Shin HR, Kim JW, Lee HP. Cervical cancer incidence and survival in Korea: 1993-2002. Int J Gynecol Cancer. 2006. 16:1833–1838.
3. Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002. 55:244–265.
4. Kim MA, Oh JK, Kim BW, Chay D, Park DC, Kim SM, Kang ES, Kim JH, Cho CH, Shin HR, et al. Prevalence and seroprevalence of low-risk human papillomavirus in Korean women. J Korean Med Sci. 2012. 27:922–928.
5. Molina R, Filella X, Auge JM, Bosch E, Torne A, Pahisa J, Lejarcegui JA, Rovirosa A, Mellado B, Ordi J, et al. CYFRA 21.1 in patients with cervical cancer: comparison with SCC and CEA. Anticancer Res. 2005. 25:1765–1771.
6. Urquiza M, Guevara T, Espejo F, Bravo MM, Rivera Z, Patarroyo ME. Two L1-peptides are excellent tools for serological detection of HPV-associated cervical carcinoma lesions. Biochem Biophys Res Commun. 2005. 332:224–232.
7. Nguyen GK, Nguyen-Ho P, Husain M, Husain EM. Cervical squamous cell carcinoma and its precursor lesions: cytodiagnostic criteria and pitfalls. Anat Pathol. 1996. 1:139–164.
8. Orsmark C, Skoog T, Jeskanen L, Kere J, Saarialho-Kere U. Expression of allograft inflammatory factor-1 in inflammatory skin disorders. Acta Derm Venereol. 2007. 87:223–227.
9. Iris FJ, Bougueleret L, Prieur S, Caterina D, Primas G, Perrot V, Jurka J, Rodriguez-Tome P, Claverie JM, Dausset J, et al. Dense Alu clustering and a potential new member of the NF kappa B family within a 90 kilo-base HLA class III segment. Nat Genet. 1993. 3:137–145.
10. Kimura M, Kawahito Y, Obayashi H, Ohta M, Hara H, Adachi T, Tokunaga D, Hojo T, Hamaguchi M, Omoto A, et al. A critical role for allograft inflammatory factor-1 in the pathogenesis of rheumatoid arthritis. J Immunol. 2007. 178:3316–3322.
11. Liu S, Tan WY, Chen QR, Chen XP, Fu K, Zhao YY, Chen ZW. Daintain/AIF-1 promotes breast cancer proliferation via activation of the NF-kappaB/cyclin D1 pathway and facilitates tumor growth. Cancer Sci. 2008. 99:952–957.
12. Vartiainen MK, Machesky LM. The WASP-Arp2/3 pathway: genetic insights. Curr Opin Cell Biol. 2004. 16:174–181.
13. Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004. 64:8585–8594.
14. Semba S, Iwaya K, Matsubayashi J, Serizawa H, Kataba H, Hirano T, Kato H, Matsuoka T, Mukai K. Coexpression of actin-related protein 2 and Wiskott-Aldrich syndrome family verproline-homologous protein 2 in adenocarcinoma of the lung. Clin Cancer Res. 2006. 12:2449–2454.
15. Otsubo T, Iwaya K, Mukai Y, Mizokami Y, Serizawa H, Matsuoka T, Mukai K. Involvement of Arp2/3 complex in the process of colorectal carcinogenesis. Mod Pathol. 2004. 17:461–467.
16. Zheng HC, Zheng YS, Li XH, Takahashi H, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Arp2/3 overexpression contributed to pathogenesis, growth and invasion of gastric carcinoma. Anticancer Res. 2008. 28(4B):2225–2232.
17. Bae SM, Lee CH, Cho YL, Nam KH, Kim YW, Kim CK, Han BD, Lee YJ, Chun HJ, Ahn WS. Two-dimensional gel analysis of protein expression profile in squamous cervical cancer patients. Gynecol Oncol. 2005. 99:26–35.
18. Godbout R, Bisgrove DA, Shkolny D, Day RS 3rd. Correlation of B-FABP and GFAP expression in malignant glioma. Oncogene. 1998. 16:1955–1962.
19. Custer RP, Sorof S. Target polypeptide of a carcinogen is associated with normal mitosis and carcinogen-induced hyperplasias in adult hepatocytes. Proc Natl Acad Sci U S A. 1984. 81:6738–6742.
20. Das R, Hammamieh R, Neill R, Melhem M, Jett M. Expression pattern of fatty acid-binding proteins in human normal and cancer prostate cells and tissues. Clin Cancer Res. 2001. 7:1706–1715.
21. Takahashi M, Rhodes DR, Furge KA, Kanayama H, Kagawa S, Haab BB, Teh BT. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci U S A. 2001. 98:9754–9759.
22. Teratani T, Domoto T, Kuriki K, Kageyama T, Takayama T, Ishikawa A, Ozono S, Nozawa R. Detection of transcript for brain-type fatty Acid-binding protein in tumor and urine of patients with renal cell carcinoma. Urology. 2007. 69:236–240.
23. Kebache S, Cardin E, Nguyen DT, Chevet E, Larose L. Nck-1 antagonizes the endoplasmic reticulum stress-induced inhibition of translation. J Biol Chem. 2004. 279:9662–9671.
24. Cardin E, Larose L. Nck-1 interacts with PKR and modulates its activation by dsRNA. Biochem Biophys Res Com. 2008. 377:231–235.
25. Mauri P, Scarpa A, Nascimbeni AC, Benazzi L, Parmagnani E, Mafficini A, Della Peruta M, Bassi C, Miyazaki K, Sorio C. Identification of proteins released by pancreatic cancer cells by multidimensional protein identification technology: a strategy for identification of novel cancer markers. FASEB J. 2005. 19:1125–1127.
26. Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK J, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996. 110:1975–1980.
27. Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993. 7:331–342.
28. Bahnassy AA, Zekri AR, Saleh M, Lotayef M, Moneir M, Shawki O. The possible role of cell cycle regulators in multistep process of HPV-associated cervical carcinoma. BMC Clin Pathol. 2007. 7:4.
29. Nichols GE, Williams ME, Gaffey MJ, Stoler MH. Cyclin D1 gene expression in human cervical neoplasia. Mod Pathol. 1996. 9:418–425.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download