Abstract
Telomerase play a key role in the maintenance of telomere length and chromosome integrity. We have evaluated the association between telomerase activity and the risk of lung cancer in peripheral blood. Telomerase activity in peripheral blood mononuclear cells was measured by a PCR-designed telomeric repeat amplification protocol in 63 lung cancer patients and 190 healthy controls that were matched for age, gender, and smoking status. Telomerase activity was significantly lower in the lung cancer patients than in controls (mean ± standard deviation; 1.32 ± 1.65 vs 2.60 ± 3.09, P < 1 × 10-4). When telomerase activity was categorized into quartiles based on telomerase activity in the controls, the risk of lung cancer increased as telomerase activity reduced (Ptrend = 1 × 10-4). Moreover, when the subjects were categorized based on the median value of telomerase activity, subjects with low telomerase activity were at a significantly increased risk of lung cancer compared to subjects with high telomerase activity (adjusted odds ratio = 3.05, 95% confidence interval = 1.60-5.82, P = 7 × 10-4). These findings suggest that telomerase activity may affect telomere maintenance, thereby contributing to susceptibility to lung cancer.
Telomeres consist of long, repetitive sequences of TTAGGG and an associated telomere-binding protein complex (shelterin) (1). The telomeres of somatic cells progressively shorten with each mitotic division, owing to the inability of DNA polymerase to fully replicate the 3' end of the DNA strand. Telomeres function to cap the ends of chromosomes and protect chromosomes from degradation, end-to-end fusion, and irregular recombination. Thus, progressive telomere shortening interferes with the formation of telomeric caps, which ultimately leads to chromosomal instability and can increase tumor formation by increasing the rate of mutation of oncogenes and tumor suppressor genes (2-5). Telomerase, a RNA-dependent DNA polymerase that consists of a template RNA (TERC) and a catalytic reverse transcriptase (TERT), adds telomeric DNA repeats de novo after each cell division, thus maintaining telomere length and function despite the telomere attrition that normally occurs during chromosomal replication (6, 7).
Several studies have documented considerable variation in telomere length and telomerase activity of peripheral blood lymphocytes among healthy individuals of the same age (8, 9). The heritability of telomere length has been linked to regions on chromosomes 3, 10, 14, and 18 (10-13). In addition, several cross-sectional and prospective studies have observed that individuals who had shorter telomeres are at an increased risk for the development of a variety of human cancers, including lung cancer (14, 15). Considering these observations, we have hypothesized that the heritable variation of telomerase activity may affect the risk of lung cancer. To test this hypothesis, we evaluated the association between telomerase activity of peripheral blood mononuclear cells (PBMCs) and lung cancer risk in a case-control study.
This case-control study included 63 patients with lung cancer and 190 healthy controls. The cases were recruited from patients who were newly diagnosed with primary lung cancer between January and July 2004 at Kyungpook National University Hospital, in Daegu, Korea. The control subjects were randomly selected from a pool of healthy volunteers who visited the general health check-up center at Kyungpook National University Hospital during the same period. We randomly selected 190 control subjects that were matched to the cases based on age ( ± 5 yr), gender, and smoking status. All of the cancer patients and the controls were ethnic Koreans who resided in Daegu City or the surrounding regions.
Changes in telomerase activity were measured in PBMCs from lung cancer patients and healthy controls. PBMCs were separated from serum and red blood cells by gradient density centrifugation through Ficoll-Paque (GF Health Care, Piscataway, NJ, USA) and stored in liquid nitrogen. The frozen lymphocytes were thawed and cultured in RPMI 1640 media supplemented with 20% heat-inactivated fetal bovine serum and 112.5 µg/mL of phytohemagglutinin. Cells were incubated at 37℃ for 72 hr in a humidified atmosphere containing 5% CO2. PBMCs proteins were obtained by adding lysis buffer and centrifuged at 16,000 g for 10 min at 4℃. The protein concentration was measured using the Bio-Rad Protein Assay (Bio-Rad, Richmond, CA, USA).
Telomerase activity in PBMCs was detected using a quantitative telomerase detection kit (Allied Biotech, Inc., Germantown, MD, USA) according to the manufacturer's protocol, which is based on a PCR-designed telomeric repeat amplification protocol. Briefly, telomerase in the cell extract from 1 × 105 cells of PBMCs added telomeric repeats (TTAGGG) onto the 3' end of the substrate oligonucleotide and QTD premix (Allied Biotech), and was amplified with a LightCycler480 machine (Roche Applied Science, Indianapolis, IN, USA). The generated PCR products are directly detected by measuring the increase in fluorescence caused by binding of SYBR Green to double-stranded DNA. A heat-inactivated cell extract served as a negative control. The real-time PCR conditions were as follows: telomerase reaction for 20 min at 25℃, PCR initial activation step for 10 min at 95℃, followed by 45 cycles of denaturation for 15 sec at 95℃, annealing for 15 sec at 60℃, extension for 15 sec at 72℃.
Telomerase activity was analyzed as continuous and categorial variables. ANOVA or a t-test was used to evaluate the differences in telomerase activity as a continuous variable by case-control status, age, gender, and smoking history (never-, or ever-smoker). As a categorical variable, the quartile value of telomerase activity, according to the distribution of telomerase activity in control subjects, was used to compare the differences between the cases and controls. In addition, telomerase activity was dichotomized at the median value in control subjects. The categorized telomerase activity of the cases and controls were compared using a chi-squared test. Unconditional logistic regression analysis was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs), with adjustment for possible confounders (gender and smoking status as nominal variables, and age and pack-years of smoking as continuous variables). The homogeneity test was conducted to compare the difference between telomerase activity-related OR of different groups. All of the analyses were performed using Statistical Analysis Software for Windows, version 8.12 (SAS institute, Cary, NC, USA).
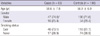
The demographics of the cases and controls enrolled in the current study are shown in Table 1. There were no significant differences in the distribution of mean age, gender, or smoking status between the cases and controls; thus, adequate matching was conducted based on these three variables. However, the number of pack-years in ever-smokers was significantly higher in the cases than in the controls (mean ± standard deviation (SD): 36.4 ± 17.6 vs 26.4 ± 11.9 pack-years; P < 0.001).
Telomerase activity was significantly lower in lung cancer patients than in healthy controls (mean ± SD: 1.31 ± 1.65 vs 2.60 ± 3.09, P < 1 × 10-4). The effects of covariates on telomerase activity in the cases and controls are shown in Table 2. There were no significant differences in telomerase activity according to age, gender and smoking status in the case or control groups. When telomerase activity in the case group was compared with the control group, telomerase activity was significantly lower in the cases than the controls for each of the subgroups evaluated, age, gender, and smoking status.
Table 3 shows the risk of lung cancer related to telomerase activity. When the subjects were categorized into quartiles of telomerase activity based on the telomerase activity distribution of the controls, with the fourth (highest) quartile used as the reference category, the adjusted OR for lung cancer was increased from 1.65 (95% CI, 0.55-4.96) to 3.38 (95% CI, 1.23-9.26) to 4.74 (95% CI, 1.77-12.71) as the telomerase activity decreased from the 3rd to the 1st quartile (Ptrend = 6 × 10-4), respectively. When the median value of telomerase activity in the control subjects was used as the cut-off between high and low telomerase activity, individuals with low telomerase activity were at a significantly increased risk of lung cancer compared to subjects with high telomerase activity (adjusted OR, 3.05; 95% CI, 1.60-5.82; P = 7 × 10-4).
The effect of telomerase activity on the risk of lung cancer was further examined after stratifying the subjects according to age, gender, smoking status, and tumor histology. When the subjects were stratified by the median age, the effect of telomerase activity on the risk of lung cancer was significant in younger individuals (adjusted OR, 7.96; 95% CI, 2.20-28.68; P = 0.01), but not in older individuals (adjusted OR, 1.58; 95% CI, 0.70-3.55; P = 0.27; P value of test for homogeneity [PH] = 0.04) (Table 4). When stratified by gender and smoking status, the effect of low telomerase activity on the risk of lung cancer was not significantly different between males and females, as well as never- and ever-smokers (PH = 0.62 and 0.98, respectively). When the analysis was stratified by tumor histology, the effect of low telomerase activity was significant for non-small cell lung cancer (adjusted OR, 3.92; 95% CI, 1.62-6.53; P = 0.001), but not for small cell lung cancer (adjusted OR, 2.46; 95% CI, 0.62-9.75; P = 0.20, PH = 0.72) (Table 4).
In this study, we investigated the association between the telomerase activity of PBMCs and the risk of lung cancer. We showed that individuals with low telomerase activity were at a significantly increased risk of lung cancer, and that the risk of lung cancer increased as the telomerase activity decreased. These findings suggest that telomerase activity may affect telomere maintenance, thereby contributing to the susceptibility to lung cancer.
Telomerase is overexpressed in the vast majority of human cancers (16, 17). Because telomerase maintains telomeres, the finding of high telomerase activity in cancers might lead to the hypothesis that longer inherited telomere length is causally related to human cancer (5, 18, 19). However, in contrast to this hypothesis, studies of telomerase knockout mice found that telomere shortening induces chromosome instability, which is perpetuated through fusion-bridge-breakage cycles that increases the risk of cancer development (20-23). Moreover, several studies have observed that individuals with shorter telomeres are at an increased risk for the development of various human cancers (14, 15, 24). In the present study, we found that low telomerase activity in PBMCs was associated with a significantly increased risk of lung cancer. This finding suggests that low telomerase activity may lead to impaired telomere length maintenance, which would increase the risk of lung cancer. Our finding corroborates previous observations which have demonstrated a link between shorter telomere length and cancer (14, 15, 20-24).
Recently, a genome-wide association study has shown that a chromosomal region (5p15.33) that contains TERT, a major determinant of telomerase activity, contributes to the susceptibility to lung cancer (25). In addition, polymorphism(s) in the promoter of the TERT gene have been shown to affect TERT expression, and thereby modulate telomere length and lung cancer risk (26-28). Furthermore, common variants near TERC, which encodes the telomerase RNA component, have been shown to be associated with telomere length in a genome-wide association analysis (12). These studies indicate that telomerase activity may affect telomere length, and thereby influence the risk of lung cancer, further supporting our finding of an association between telomerase activity and the risk of lung cancer.
An interesting finding of the current study is that telomerase activity had a more pronounced association in younger than in older individuals. Although the reason for the observed age-dependent difference in the risk conferred by telomerase activity remains to be elucidated, this difference may be attributed to age-related changes in telomere length and telomerase activity. It has been shown that telomere length and telomerase activity decrease in a linear fashion with age, and the levels are significantly lower in older people (5, 25, 26). Therefore, it is possible that the effect of variation in telomerase activity on telomere length may be less in older individuals than in younger individuals. Several studies have observed a similar finding; specifically, the effect of inheriting telomere length on the risk of lung cancer was more pronounced in younger subjects than in older subjects (14, 15, 24). The current study, along with previous published studies investigating the relationship between telomere length and lung cancer risk (14, 15, 24), suggest that telomere dysfunction may play a greater role in the development of lung cancer in younger individuals than older individuals. However, it is possible that our finding was due to chance because of the relatively small number of subjects in the subgroups. Therefore, larger studies should be conducted to confirm this finding.
A number of limitations in the present study need to be addressed. One such limitation was the small sample size, which did not allow for validation testing in a separate cohort or for reliable subgroup analysis. Therefore, our results need to be replicated to validate the observed association. Furthermore, the age ranges in our studies were limited, reducing our ability to observe a significant correlation with increasing age and decreasing telomerase activity. In addition, one must consider potential biases that might influence the results of hospital-based case-control studies, primarily selection and information biases.
In conclusion, we showed that low telomerase activity in PBMCs is associated with a significantly increased risk of lung cancer. It is interesting to note that in the current study, we demonstrated that the effect of low telomerase activity on the risk of lung cancer was more pronounced in younger individuals than older individuals. These results suggest that variation in telomerase activity may contribute to a genetic predisposition to lung cancer by influencing telomere length maintenance. However, this is the first case-control study investigating the association between telomerase activity and the risk of cancer. Therefore, additional studies are required to confirm the findings herein.
Figures and Tables
Notes
This study is supported in part by a grant from the national R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0720550-2), and Conversing Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education Science and Technology (2009-0093681).
References
1. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Gene Dev. 2005. 19:2100–2110.
2. Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999. 97:527–538.
3. Blasco MA. Telomeres and human disease: aging, cancer and beyond. Nat Rev Genet. 2005. 6:611–622.
4. Calado RT, Young NS. Telomere disease. N Engl J Med. 2009. 361:2353–2365.
5. Eisenberg DT. An evolutionary review of human telomere biology: the thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011. 23:149–167.
6. Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989. 59:521–529.
7. Shay JW, Zou Y, Hiyama E, Wright WE. Telomerase and cancer. Hum Mol Genet. 2001. 10:677–685.
8. Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Yahata N, Ando K, Toyama K, Hoshika A, Takasaki M, Mori M, Shay JW. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum Genet. 1998. 102:397–402.
9. Bischoff C, Graakjaer J, Petersen HC, Hjelmborg JV, Vaupel JW, Bohr V, Koelvraa S, Christensen K. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005. 8:433–439.
10. Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006. 78:480–486.
11. Mangino M, Richards JB, Soranzo N, Zhai G, Aviv A, Valdes AM, Samani NJ, Deloukas P, Spector TD. A genome-wide association study identifies a novel locus on chromosome 18q12.2 influencing white cell telomere length. J Med Genet. 2009. 46:451–454.
12. Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G, Valdes AM, Blackburn H, Mateo Leach I, de Boer RA, Kimura M, Aviv A, Goodall AH, Ouwehand W, van Veldhuisen DJ, van Gilst WH, Navis G, Burton PR, Tobin MD, Hall AS, Thompson JR, Spector T, Samani NJ. Wellcome Trust Case Control Consortium. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010. 42:197–199.
13. Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, Bis JC, Fitzpatrick AL, Smith E, Johnson AD, Gardner JP, Srinivasan SR, Schork N, Rotter JI, Herbig U, Psaty BM, Sastrasinh M, Murray SS, Vasan RS, Province MA, Glazer NL, Lu X, Cao X, Kronmal R, Mangino M, Soranzo N, Spector TD, Berenson GS, Aviv A. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010. 107:9293–9298.
14. Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, Kim CH, Kam S, Jung TH, Park JY. Telomere length and the risk of lung cancer. Cancer Sci. 2008. 99:1385–1389.
15. Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010. 304:69–75.
16. Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994. 266:2011–2015.
17. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997. 33:787–791.
18. Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992. 27:383–389.
19. Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001. 11:S27–S31.
20. Blasco MA, Lee HW, Hande MP, Samper E, Landsdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997. 91:25–34.
21. Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999. 96:701–712.
22. Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001. 28:155–159.
23. Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000. 406:641–645.
24. Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003. 95:1211–1218.
25. McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, McLaughlin J, Shepherd F, Montpetit A, Narod S, Krokan HE, Skorpen F, Elvestad MB, Vatten L, Njølstad I, Axelsson T, Chen C, Goodman G, Barnett M, Loomis MM, Lubiñski J, Matyjasik J, Lener M, Oszutowska D, Field J, Liloglou T, Xinarianos G, Cassidy A, Vineis P, Clavel-Chapelon F, Palli D, Tumino R, Krogh V, Panico S, González CA, Ramón Quirós J, Martínez C, Navarro C, Ardanaz E, Larrañaga N, Kham KT, Key T, Bueno-de-Mesquita HB, Peeters PH, Trichopoulou A, Linseisen J, Boeing H, Hallmans G, Overvad K, Tjønneland A, Kumle M, Riboli E, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. EPIC Study. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008. 40:1404–1406.
26. Hsu CP, Hsu NY, Lee LW, Ko JL. Ets2 binding site single nucleotide polymorphism at the hTERT gene promoter: effect on telomerase expression and telomere maintenance in non-small cell lung cancer. Eur J Cancer. 2006. 42:1466–1474.
27. Matsubara Y, Murata M, Yoshida T, Watanabe K, Saito I, Miyaki K, Omae K, Ikeda Y. Telomere length of normal leukocytes is affected by a functional polymorphism of hTERT. Biochem Biophys Res Commun. 2006. 341:128–131.
28. Choi JE, Kang HG, Jang JS, Choi YY, Kim MJ, Kim JS, Jeon HS, Lee WK, Cha SI, Kim CH, Kam S, Jung TH, Park JY. Polymorphisms in telomere maintenance genes and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2009. 18:2773–2781.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download