Abstract
This study was conducted to determine if nasal salmon calcitonin has additional beneficial effects on clinical symptoms, serum NO, IL-1β, matrix metalloproteinase 3, urinary C-terminal telopeptide type II collagen (CTX-II) levels and MRI findings in knee osteoarthritis (OA) when used concomitantly with exercise therapy. Fifty female patients with knee OA were randomized into two groups. The first group (n = 30) received 200 IU/day nasal salmon calcitonin and a home exercise program; the second group (n = 20) received a home exercise program for 6 months. Compared with baseline,while significant improvements were observed in visual analogue scale (VAS), WOMAC pain, physical function scores, 20-m walking time (P < 0.001) and WOMAC stiffness score (P = 0.041) in the first group, walking and resting VAS, and WOMAC physical function scores were improved (P = 0.029) in the second group after treatment. Significantly increased levels of serum NO and urinary CTX-II (P < 0.001) and significant improvements in the area of medial femoral condyle (P < 0.05) were noted only in the first group. There were significant differences in VAS activation values (P = 0.032) and NO levels (P < 0.001) in the favor of the first group. In conclusion, nasal salmon calcitonin may have possible chondroprotective effects besides its known effects on symptoms in patients with knee OA.
Osteoarthritis (OA) is a chronic degenerative disease characterized by the loss of cartilage in the joints covered by synovia. Matrix homeostasis of the cartilage is maintained by the balance of synthesis and degeneration of the matrix, which is produced by chondrocytes. Both synthesis and degeneration are under the control of messenger proteins, growth factors and cytokines. While growth factors induce the production of connective tissue in general, cytokines lead to the matrix destruction (1). Major cytokines triggering the cartilage destruction are Interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α), both of which are secreted from synovial macrophages. Interleukin 1β decreases the prostaglandin synthesis, impairs repair of the cartilage matrix damage and facilitates the secretion of the matrix metalloproteinase (MMPs). In particular, MMP-3 has a major role in OA (2). On the other hand, several cytokines, including NO, are secreted from chondrocytes with the stimulation of IL-1β. Moreover, increased expression of NO and thus increased ratio of nitrite/nitrate were found in OA (1).
Collagen type II (CTX-II) is the main structural component of the cartilage tissue. It was shown that urinary excretion of the degradation products of CTX-II is increased in patients with OA and it correlates with the progression of articular damage (3).
Experimental and clinical observations suggest that structural integrity of the articular cartilage depends on normal subchondral bone turnover and healthy function of chondrocytes, as well as normal biomechanical stresses (4). Degenerative process in OA is not limited to the cartilage. Given that there is a strong relationship between subchondral bone and articular cartilage, an optimal therapeutic agent should regulate the metabolic activity of both the bone and the cartilage (4). There are some studies carried out and still ongoing on preventing the OA-associated articular damage and developing an effective pharmacological agent for the treatment of OA. Antiresorptive agents have been shown to act both on bone and cartilage and they were proposed to be considered as OA-modifying agents (5).
Recent in vivo and in vitro experiments indicated that calcitonin, an antiresorptive agent, acts both on cartilage and subchondral bone, decreases the severity of the cartilage lesions in OA, and involves in altered biochemical content and supramolecular organization of the cartilage matrix (6-9). Moreover, calcitonin showed an analgesic effect on bone pain associated with osteoporotic vertebral fractures, Paget's disease or other musculoskeletal disorders (10).
Exercise is a widely used non-pharmacological treatment modality for OA and it was found effective in a vast number of studies (11, 12). However, given that calcitonin has analgesic and beneficial effects on cartilage and subchondral bone, we hypothesized that it may be a potential therapeutic option for the treatment of OA. Therefore, we aimed to determine if nasal salmon calcitonin treatment has additional beneficial effects on clinical symptoms, cartilage degeneration-associated biochemical parameters, and magnetic resonance image (MRI) findings in patients with knee OA when used concomitantly with the exercise therapy.
Female patients with the diagnosis of primary knee OA according to the ACR criteria were included to the study. All patients were postmenopausal. Informed consent was obtained from all patients. Kellgren Lawrence radiological stage II or III patients with ACR criteria-based bilateral OA and knee pain were included to the study. Patients with a previous lower extremity surgery, intraarticular injections or physical therapy for knee within the last year, any previous calcitonin treatment, allergy to calcitonin, peripheral or central neurological disorder, or severe cardiac disease and incorporated patients were excluded from the study.
After a detailed anamnesis and physical examination, complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and rheumatoid factor (RF) levels were measured and the tests of liver and kidney functions were performed in all patients included to the study. In addition, conventional standing anteroposterior (AP) and lateral knee radiographies were taken.
The present study was designed as a prospective, randomized, single-blind and controlled clinical study. At the beginning of the study, the first and second groups consisted of 32 and 23 patients, respectively. However, 2 patients from the first group and 3 from the second group were lost at follow-up, leaving 30 and 20 patients respectively in each group.
The first group of patients (n = 30) received 200 IU/day nasal salmon calcitonin (Miacalcic nasal 200, Novartis, Basel, Switzerland) by inhalation and a home exercise program, and the second group (n = 20) received a home exercise program for 6 months. Home exercise program was given in the form of an illustrated brochure. Quadriceps isometric and isotonic strengthening, joint range of motion (ROM), and hamstring stretching exercises were planned as once daily, with 2 sets of 20 repetitions and 2 min rests between the sets. Proper practical instructions were given to all patients. During the 6 months follow-up, compliance of the patients to the treatment program was assessed by weekly phone calls.
All patients were evaluated before and after the treatment protocol by a blinded investigator with respect to the following parameters. Visual Analogue Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and 20-m walking time were used for the clinical assessment. The pain at rest, walking and activation were assessed from 0 (no pain) to 10 (worst possible pain) on VAS scale. 20-m walking time was assessed with having all patients walking on the same straight ground, and using the same standard words and the same chronometer. The WOMAC consists of 24 items divided into 3 subscales: pain, stiffness and physical function. Global score has a range of 0 (no symptom) to 96 (worst symptoms), with standardizing the score to have a range of 0 to 100.
From all patients a 6-7 mL of blood sample was taken twice, before and after the treatment protocol. After centrifugation of the blood, serum samples were collected and stored at -80°. Nitric oxide, IL-1β and MMP-3 levels were determined in serum samples.
Nitric oxide levels were measured manually by Griess Reaction Assay (Shimadzu UV-1201, Kyoto, Japan). In vivo stabile end-products of the NO are nitrite and nitrate, the levels of which are used as an index of NO production. The levels of these end-products based on cadmium reaction were determined spectrophotometrically at a wavelength of 545 nm and NO levels were calculated in µM/L. Interleukin 1β levels were measured using a chemiluminescence assay (IMMULITE, Siemens Healthcare Diagnostics GmbH, Erlangen, Germany). Serum MMP-3 levels were analyzed using a quantitative sandwich ELISA according to the manufacturer's instructions (R&D Systems Inc, Minneapolis, USA). Absorbency of the samples read at 450 nm; MMP-3 levels were automatically calculated by the device and expressed as ng/mL. CTX-II levels in the 24 hr urine samples were measured with using a commercially available ELISA kit (Immundiagnostik AG, Bensheim, Germany); the results divided by the serum creatinine concentration and were expressed as ng/mM.
Magnetic resonance images (MRIs) of the knees with more significant symptoms were obtained from all patients, with employing a cartilage sequence. All the analyses were performed on a 1.5 Tesla MRI unit (VB33D Vision Plus; Siemens, Erlangen, Germany) using an extremity coil for signal reception and delivery. FS 3D FLASH MRI parameters were defined as TR/TE: 60/11 and FA: 40°; and gradient-echo images were obtained in axial, sagittal and coronal planes. Wide slice thickness was 65 mm in axial, 70 mm in sagittal and 80 mm in coronal planes. Slice thickness was 2.5 mm for axial, sagittal and coronal planes. An imaging area of 25 cm, a spatial resolution of 1.2 × 1 mm and a matrix of 256 × 192 were used to minimize the artifacts from the contralateral knee. Sequence imaging time was 6.28 min and fat suppression prolonged the total scan time about 3 min.
MRI scans were read by a blinded musculoskeletal radiologist. In all patients, 4 articular surfaces were identified on knee: medial and lateral femoral condyles (MFC and LFC), and medial and lateral tibial plateaus (MTP and LTP). Each single plane was evaluated and graded for the presence and appearance of articular cartilage defects using a standard arthroscopic grading scheme adapted to MR imaging (SPGR). In the case of the multiple cartilage defects with different grades on any articular surface, the highest grade was considered as the grade of the cartilage defect (Table 1) (13).
Statistical procedures were performed using PASW 18.0 and SigmaStat 3.5 software. Normally distributed continuous quantitative data were expressed as n, mean and standard deviation; Kolmogorov-Smirnov test were used to for normality test and not normally distributed ones as n, median (25%-75% quartile); and qualitative data as n. Repeated and independent measurements with not normally distributed variables were analyzed by using Wilcoxon, Mann-Whitney U test and those with normally distributed variables were analyzed by using t test. Chi-square analyses were used for independent categorical variables and Marginal Homogeneity test for dependent categorical variables.
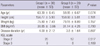
Two patients from the first group and three from the second group were lost at follow-up and data of these patients were not included to the analysis. Demographical characteristics of the patients did not differ significantly between the two groups (P = 0.074 between P = 0.807) (Table 2).
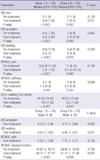
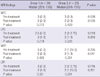
Before the treatment, there were no differences between the groups in clinical parameters, biochemical analysis and MRI findings. At the post treatment evaluation, significant improvements were noted in walking, activity and resting VAS scores, WOMAC pain and physical function scores, and 20-m walking time (P < 0.001) and in WOMAC stiffness score (P = 0.041) of the patients in the first group. On the other hand, walking and resting VAS scores, and WOMAC physical function score (P = 0.029) were significantly improved compared to the pretreatment status in patients of the second group (Table 3). Significant increases in serum NO and urinary CTX-II levels (P < 0.001) and significant improvements in MFC area (P = 0.012) were found only in the first group of patients (Tables 4, 5). Comparison of the groups revealed significant differences in VAS activity scores (P = 0.032) and serum NO levels (P < 0.001) in the favor of the first group (Tables 3, 4).
The aim of the OA treatment was to decrease the pain severity and functional limitation, as well as to slow the progression of the cartilage damage. The present study was conducted to determine if nasal salmon calcitonin treatment has additional beneficial effects when used concomitantly with the exercise therapy. In patients included to the present study, although exercise therapy had beneficial effects on the symptoms of knee OA, concomitant use of the nasal salmon calcitonin resulted in a better improvement. With improvements observed in the biochemical parameters related to chondroprotection and cartilage degeneration, nasal salmon calcitonin was considered to be effective in the treatment of knee OA.
Osteoarthritis results in pain, decreased movement, articular instability and muscle atrophy, all of them impairing the quality of life. As a result of pain, patients usually tend to limit their physical activities (14). On the other hand, calcitonin is suggested to have analgesic effects. Several experimental and clinical studies established the analgesic effects of calcitonin via central serotonergic system and increased serum beta endorphin levels (15-17). Therefore, it can be proposed that beneficial effects of calcitonin on clinical symptoms of the patients with OA may be related to its analgesic properties.
Salmon calcitonin has been found effective in the treatment of OA patients in a limited number of clinical studies (9, 18). Manicourt et al.(9) evaluated the effectiveness of 0.5 or 1.0 mg oral calcitonin preparation on 41 patients with knee OA in a randomized, double-blinded, placebo-controlled study. Authors found significant improvements in pain and functional ability in the two groups of patients receiving salmon calcitonin. Similarly, 200 IU nasal calcitonin was also reported to have beneficial effects on pain scores of the patients with knee osteoarthritis (18). Our patients treated with calcitonin also showed significant improvements in pain severity, all VAS scores assessing the functional status, WOMAC scores, and 20-m walking time.
Calcitonin has potent antiresorptive effects by directly acting on osteoclasts. Calcitonin receptors have been also identified on chondrocytes (7, 19-21). In vitro and in vivo studies demonstrated that calcitonin increases the synthesis of proteoglycans and glycosaminoglycans, increases the production of collagen II, stimulates growth of the cartilage, prevents cartilage erosions, and decreases the proteolytic and metalloproteinase activities (7, 21-23). In addition to the beneficial effects on cartilage, calcitonin also decreases subchondral bone resorption and increases the trabecular thickness (26). Thus, calcitonin is thought to have a chondroprotective effect.
In the present study, serum NO, IL-1β, and MMP-3 levels and urinary CTX-II levels were measured and the changes in the thickness of cartilage were evaluated on MRI scans in order to assess the chondroprotective effects of calcitonin. Collagen type II is the main component of the cartilage. Several studies demonstrated increased urinary excretion of CTX-II degradation products in patients with OA, as well as correlation of these products with the severity of OA (25, 26). In a placebo-controlled study, salmon calcitonin treatment significantly decreased 24-hr urinary CTX-II excretion dose-dependently after 3 months of treatment (27). Another placebo-controlled study comparing the effects of 0.5 and 1.0 mg oral salmon calcitonin in patients with knee OA reported significantly decreased CTX-II levels in patients receiving 1 mg oral calcitonin (9). In a similar designed placebo-controlled study, patients received twice daily 0.6 or 0.8 mg oral calcitonin for 14 days and authors reported decreased urinary excretion of CTX-II in patients receiving 0.8 mg oral calcitonin (28). Consistent with other studies, our results also confirmed that calcitonin treatment decreases urinary excretion of CTX-II.
Nasal calcitonin administered by inhalation at a dose of 200 IU did not alter serum IL-1β and MMP-3 levels. While, there are no studies evaluating the effects of calcitonin on IL-1β levels. Manicourt et al.(9) reported that 1 mg oral calcitonin treatment resulted in decreased MMP-3 levels. This discrepancy from our results may be due to using a different dose and preparation of calcitonin in that study.
On the other hand, 200 IU nasal calcitonin treatment decreased serum NO levels in patients with OA. Nitric oxide is one of the major catabolic factors synthesized in chondrocytes. NO was shown to inhibit collagen and proteoglycan synthesis, to increase the activation of MMPs, and to increase apoptosis and sensitivity to oxidants such as hydrogen peroxide. Because NO increases the bone resorption and contributes to the alterations in subchondral bone, it is deemed to have a role in the pathogenesis of OA (29). Literature is lacking about the effects of calcitonin treatment on serum NO levels. The results of the present study suggest that 200 IU nasal calcitonin might have beneficial effects of on subchondral bone.
The effects of nasal CT on OA is evaluated with MRI scans and some beneficial effects on cartilage defects were observed in the MFC area. Although a linear relationship was reported between urinary excretion of CTX-II and MRI findings of the cartilage defect in patients with OA (30), there are no studies evaluating the effects of calcitonin on MRI findings of OA. The beneficial effects of calcitonin on MRI findings of OA in the present study was thought to be related to the fact that calcitonin decreases the degradation of calcitonin, a major component of the cartilage.
Although, it is well known that calcitonin is beneficial on symptoms of OA, to our knowledge, this is the first study evaluating the effects of calcitonin on cartilage degradation-related mediators as well as on MRI findings when used concomitantly with the exercise therapy. Limitations of the present study are small sample size and a short follow-up time. Despite considerable advancements in the treatment of OA, disease-modifying and chondroprotective treatment modalities are still under investigation. Many newer potential agents are being tested in preclinical trials and the results of the clinical studies are yet insufficient to draw a clear conclusion. Although nearly 10 yr passed since the disease-modifying drugs were first introduced, neither of them was approved by FDA for the treatment of OA.
The aim of the present study was to determine if calcitonin, which is recently shown to play a role in supramolecular organization of the cartilage matrix, has a disease-modifying effect in OA. Because the use of nasal calcitonin by inhalation concomitantly with the exercise therapy shows beneficial effects on symptoms of the disease and MRI findings of the cartilage thickness and levels of cartilage degradation-related mediators in patients with OA, it may have the potential to be a disease-modifying agent.
Figures and Tables
References
1. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010. 6:625–635.
2. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002. 39:237–246.
3. Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio Le Graverand MP, Degroot J, Slagboom PE. Urinary CTX-II levels are associated with radiographic subtypes of osteoarthritis in hip, knee, hand, and facet joints in subject with familial osteoarthritis at multiple sites: the GARP study. Ann Rheum Dis. 2006. 65:360–365.
4. Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004. 50:1193–1206.
5. Karsdal MA, Tanko LB, Riis BJ, Sondergard BC, Henriksen K, Altman RD, Qvist P, Christiansen C. Calcitonin is involved in cartilage homeostasis: is calcitonin a treatment for OA? Osteoarthritis Cartilage. 2006. 14:617–624.
6. Manicourt DH, Altman RD, Williams JM, Devogelaer JP, Druetz-Van Egeren A, Lenz ME, Pietryla D, Thonar EJ. Treatment with calcitonin suppresses the responses of bone, cartilage, and synovium in the early stages of canine experimental osteoarthritis and significantly reduces the severity of the cartilage lesions. Arthritis Rheum. 1999. 42:1159–1167.
7. Sondergaard BC, Wulf H, Henriksen K, Schaller S, Oestergaard S, Qvist P, Tankó LB, Bagger YZ, Christiansen C, Karsdal MA. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthritis Cartilage. 2006. 14:759–768.
8. Sondergaard BC, Oestergaard S, Christiansen C, Tankó LB, Karsdal MA. The effect of oral calcitonin on cartilage turnover and surface erosion in an ovariectomized rat model. Arthritis Rheum. 2007. 56:2674–2678.
9. Manicourt DH, Azria M, Mindeholm L, Thonar EJ, Devogelaer JP. Oral salmon calcitonin reduces Lequesne's algofunctional index scores and decreases urinary and serum levels of biomarkers of joint metabolism in knee osteoarthritis. Arthritis Rheum. 2006. 54:3205–3211.
10. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain Relief From Nasal Salmon Calcitonin İn Osteoporotic Vertebral Crush Fractures. Acta Orthop Scand Suppl. 1997. 275:112–114.
11. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000. 27:2215–2221.
12. Evcik D, Sonel B. Effectiveness of a home-based exercise therapy and walking program on osteoarthritis of the knee. Rheumatol Int. 2002. 22:103–106.
13. Bredella MA, Tirman PF, Peterfy CG, Zarlingo M, Feller JF, Bost FW, Belzer JP, Wischer TK, Genant HK. Accuracy of T2-weighted fast spin-echo MR imaging with fat saturation in detecting cartilage defects in the knee: comparison with arthroscopy in 130 patients. AJR Am J Roentgenol. 1999. 172:1073–1080.
14. van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: the relationship with articular, kinesiological and psychological characteristics. J Rheumatol. 1998. 25:125–133.
15. Ofluoglu D, Akyuz G, Unay O, Kayhan O. The effect of calcitonin on beta-endorphin levels in postmenopausal osteoporotic patients with back pain. Clin Rheumatol. 2007. 26:44–49.
16. Takayama B, Kikuchi S, Konno S, Sekiguchi M. An immunohistochemical study of the antinociceptive effect of calcitonin in ovariectomized rats. BMC Musculoskelet Disord. 2008. 9:164.
17. Ito A, Kumamoto E, Takeda M, Shibata K, Sagai H, Yoshimura M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J Neurosci. 2000. 20:6302–6308.
18. Badurski J, Jeziernicka E, Naruszewicz K, Racewicz A. Comparative analysis of three treatment regimens for treating gonarthritis with calcitonin, naproxen and flavonoids based on EULAR criteria and visual analogue scale (VAS). Pol Tyg Lek. 1995. 50:37–40.
19. Suzuki H, Nakamura I, Takahashi N, Ikuhara T, Matsuzaki K, Isogai Y, Hori M, Suda T. Calcitonin-induced changes in the cytoskeleton are mediated by a signal pathway associated with protein kinase A in osteoclasts. Endocrinology. 1996. 137:4685–4690.
20. Shyu JF, Shih C, Tseng CY, Lin CH, Sun DT, Liu HT, Tsung HC, Chen TH, Lu RB. Calcitonin induces podosome disassembly and detachment of osteoclasts by modulating Pyk2 and Src activities. Bone. 2007. 40:1329–1342.
21. Franchimont P, Bassleer C, Henrotin Y, Gysen P, Bassleer R. Effects of human and salmon calcitonin on human articular chondrocytes cultivated in clusters. J Clin Endocrinol Metab. 1989. 69:259–266.
22. Sondergaard BC, Madsen SH, Segovia-Silvestre T, Paulsen SJ, Christiansen T, Pedersen C, Bay-Jensen AC, Karsdal MA. Investigation of the direct effects of salmon calcitonin on human osteoarthritic chondrocytes. BMC Musculoskelet Disord. 2010. 11:62.
23. Di Nino DL, Linsenmayer TF. Positive regulation of endochondral cartilage growth by perichondrial and periosteal calcitonin. Endocrinology. 2003. 144:1979–1983.
24. Karsdal MA, Leeming DJ, Dam EB, Henriksen K, Alexandersen P, Pastoureau P. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage. 2008. 16:638–646.
25. Reijman M, Hazes JM, Bierma-Zeinstra SM, Koes BW, Christgau S, Christiansen C, Uitterlinden AG, Pols HA. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004. 50:2471–2478.
26. Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio-Le Graverand MP, DeGroot J, Slagboom PE. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study. Osteoarthritis Cartilage. 2007. 15:379–385.
27. Bagger YZ, Tankó LB, Alexandersen P, Karsdal MA, Olson M, Mindeholm L, Azria M, Christiansen C. Oral salmon calcitonin induced suppression of urinary collogen type II degradation in postmenapausal women: A new potential treatment of osteoarthritis. Bone. 2005. 37:425–430.
28. Karsdal MA, Byrjalsen I, Henriksen K, Riis BJ, Lau EM, Arnold M, Christiansen C. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthritis Cartilage. 2010. 18:150–159.
29. Lotz M. The role of nitric oxide in articular cartilage damage. Rheum Dis Clin North Am. 1999. 25:269–282.
30. Dam EB, Byrjalsen I, Karsdal MA, Qvist P, Christiansen C. Increased urinary excretion of C-telopeptides of type II collagen (CTX-II) predicts cartilage loss over 21 months by MRI. Osteoarthritis Cartilage. 2009. 17:384–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download