Abstract
Since the risk of developing allergic disease increases in individuals exposed to allergens previously, even during the neonatal period, the immunologic status of a fetus may be important in the subsequent development of allergy. We evaluated the fetal factors to predict atopic dermatitis (AD) at 12 months in 412 infants of a COhort for Childhood Origin of Asthma and Allergic Diseases (COCOA) in the general Korean population. Cord blood mononuclear cells (CBMCs) were stimulated with ovalbumin and phytohemagglutinin and cellular proliferative response and concentrations of interleukin-13 and interferon-γ, were measured. The risk of developing AD was greater in boys than girls (OR 1.97, 95% CI 1.26-3.09), infants delivered by cesarean section than vaginally (OR 1.93, 95% CI 1.14-3.26) and infants with than without parental history of AD (OR 2.34, 95% CI 1.29-4.24). The CBMC proliferative response to phytohemagglutinin stimulation was higher in infants with than without AD (P = 0.048), but no difference was observed in ovalbumin-stimulated cells (P = 0.771). Risk factors for the development of AD at 12 months include male gender, delivery by cesarean section and parental history of AD. Increased CBMC proliferative response to phytohemagglutinin stimulation may predict the development of AD at 12 months.
Although allergic diseases are increasing recently both worldwide and in Korea (1-4), the reason for these increases, their risk factors and the onset period for allergic disease have not been determined. The increased incidence of allergic diseases may result from changes in the environment rather than genetic predisposition, with many studies attempting to identify the risk factors for development of allergic diseases (2, 3, 5). Early detection and prevention of allergic diseases in the neonatal period may be enhanced by identifying environmental factors that influence fetal and post-natal development of atopic disease (6, 7).
Allergic diseases originate in response to exposure during early life, with pregnancy and early childhood being critical periods for detecting early markers of allergic disease (8, 9). An immunological bias toward atopy and asthma may be established in utero during the development of the fetal immune system (6, 10) and may be detected by assays of cord blood. Because atopic disease during early infancy may manifest as atopic dermatitis (AD), identifying factors associated with the development of AD, especially prenatally and in cord blood, may prevent the development of allergic diseases. Studies measuring IgE or mediators in cord blood and investigating their association with the development of asthma and other IgE-mediated allergic diseases, however, have yielded conflicting results (11, 12), making it difficult to establish a causal relationship between these factors and the subsequent development of AD.
Cord blood T lymphocytes have been shown to proliferate when stimulated with ovalbumin, lactoglobulin or house dust mites, suggesting that priming has already occurred (13, 14) and that the fetal immune system may be primed for the development of allergic disease before birth. It is unclear, however, whether cellular proliferative responses of cord blood will lead to the production of Th1/Th2 cytokines and allergic disorders. Defects in interferon (IFN)-γ secretion have been observed in the cord blood of individuals who later develop allergic disease and to be closely related to the cellular mechanisms underlying these diseases (15). Mediators of allergic disease development, however, have not been determined. We therefore assessed factors in newborns of the general Korean population that could affect the development of AD at 12 months. The factors that we investigated included family history of allergic disease, environmental factors, and inflammatory status of cord blood.
We constructed a COhort for Childhood Origin of Asthma and Allergic Diseases (COCOA) in the general Korean population, by recruiting healthy pregnant women who delivered at four hospitals (Asan Medical Center, Samsung Medical Center, Severance Hospital, and CHA Medical Center) beginning in December 2007. COCOA selection criteria were designed to enroll normal babies, so we excluded premature infants and those with congenital diseases. A modified questionnaire of the International Study of Asthma and Allergies in Childhood (ISAAC) was completed by each woman at 36 weeks of gestational age. This questionnaire includes questions about demographic factors in parents, parental medical histories including allergic diseases, environmental factors, and dietary pattern. The delivery record for each infant was written at birth. Cord blood samples were collected from most subjects at the time of delivery. Each woman completed the same questionnaire when their infants reached 6 and 12 months to assess demographic data, allergic disease history, environmental factors, and dietary pattern of the infants. Presence of AD was diagnosed by pediatric allergy specialists when the infants were followed-up at the hospital at 12 months.
Of the 1,294 pregnant women enrolled, 195 were excluded due to disagreement or failure to fulfill inclusion criteria. Cord blood was obtained from 790 infants. We identified 412 infants who were diagnosed with AD at 12 months by allergy specialists and for whom their parents completed questionnaires through 12 months. Finally, we analyzed the association between AD at 12 month and cord blood mediators in 287 infants.
Heparinized blood samples were obtained from full-term newborn umbilical cords at birth. White blood cells (WBC) and eosinophils were counted and total IgE levels were measured with CAP system (Pharmacia, Uppsala, Sweden). Cord blood mononuclear cells (CBMCs) were separated on a Histopaque (Sigma Chemical Co., St Louis, USA) gradient within 24 hr of sample collection, and the collected cells were washed with phosphate-buffered saline. CBMCs were cultured in Iscove's modified Dulbecco's medium supplemented with 1% antibiotic-mycotic and 10% fetal bovine serum (all from GIBCO BRL, Eggenstein, Germany). CBMCs were re-suspended to 1 × 105 cells/200 µL/well in 96-well plates and incubated with 100 µg/mL ovalbumin (OVA, chicken egg albumin grade V; Sigma Chemical Co., St Louis, MO, USA) or 10 µL/mL phytohemagglutinin (PHA)-M (GIBCO BRL) for 48 hr, with 1 µCi of [3H]-thymidine added to each well for the final 12 hr. The cells were harvested onto microfiber filters (Simport, Beloeil, Canada) and the radioactivity on the dried filters was measured in a liquid scintillation counter. All samples were assayed in triplicate.
The supernatants of PHA-stimulated cells were obtained after 48 hr in culture and stored at -70℃ until assayed for the cytokines, IL-13 and IFN-γ, by enzyme-linked immunosorbent assays (ELISA) using commercially available kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. All supernatants were assayed in duplicate. The limits of detection were 62.5 pg/mL for IL-13 and 15.6 pg/mL for IFN-γ.
Associations between log-transformed cord blood immune responses and the prevalence of AD at 12 months were estimated using chi-square tests and multiple logistic regression analyses, expressed as adjusted odds ratios (aOR) with 95% confidence intervals (CIs). Multivariate models were adjusted for child's sex, season of birth, maternal age at delivery, gestational period, maternal education level and parental allergy history. All statistical analyses were performed using SPSS 18.0 software, with a P value < 0.05 considered statistically significant.
The study protocol was approved by the institutional review board of Asan Medical Center (IRB No. 2008-0616), Samsung Medical Center (IRB No. 2009-02-021), Yonsei University (IRB No. 4-2008-0588) and CHA Medical Center (IRB No. 2010-010). Informed consent was confirmed by each IRB and obtained from the parents of each infant.
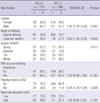
The study included 412 infants (229 boys and 183 girls) and their families (Table 1). The mean maternal age at delivery was 32.3 yr, with the time of delivery ranging from 37 to 42 weeks of gestation, and 68.0% of the infants being delivered vaginally. We found that 25.2% of these infants were born during the spring, 19.5% during the summer, 30.1% during the fall and 25.2% during the winter. Of the mothers, 23.7% had at least graduated college and 60.9% were exposed to secondhand smoke during pregnancy. We found that 15.0% of the infants had at least one parent with a history of AD, and 28.2% were diagnosed with AD at 12 months of age (Table 2).
When we assessed factors associated with the development of AD at 12 months, we found that the prevalence of AD was higher in boys than in girls (OR 1.97, 95% CI 1.26-3.09, P = 0.003) and in infants delivered by cesarean section than those delivered vaginally (OR 2.03, 95% CI 1.28-3.26, P = 0.003). There was no association, however, between AD and season of birth (P = 0.810) or maternal exposure to secondhand smoke during pregnancy (P = 0.782). Infants with a parental history of AD were at significantly higher risk of developing AD at 12 months than those without parental history (OR 2.34, 95% CI 1.29-4.24, P = 0.005). However, maternal education level was not associated with the development of AD at 12 months (P = 0.101).
The associations between AD in infants and parental history of allergic disease, including asthma, allergic rhinitis, and AD are shown in Table 3. Maternal history of asthma (P = 0.302) and allergic rhinitis (P = 0.189) was not associated with AD in infants. However, maternal history of AD was associated with AD in infants by both univariate analysis (OR 2.30, 95% CI 1.09-4.84, P = 0.029) and after adjustment (OR 3.22, 95% CI 1.14-9.08, P = 0.027). Similarly, paternal history of asthma (P = 0.837) and allergic rhinitis (P = 0.959) was not associated with AD in infants, whereas paternal history of AD was associated with the development of AD in infants when adjusted for gender, maternal age at parity, gestational age, mode of delivery, maternal education level, and season of birth (OR 5.65, 95% CI 1.97-16.21, P = 0.001).
WBC (P = 0.900) and eosinophil counts (P = 0.920), and total IgE levels (P = 0.615) in cord blood did not differ significantly between infants with and without AD (Table 4). Similarly, the cellular proliferative response of cord blood to OVA stimulation did not differ significantly between infants with and without AD (7.3 ± 1.0 cpm vs 7.8 ± 1.0 cpm, P = 0.098) (Table 4), nor did the ratio of cellular proliferative response of OVA-stimulated to -unstimulated cells between the groups with and without AD (0.6 ± 0.4 cpm vs 0.6 ± 0.5 cpm, P = 0.771) (Fig. 1).
When cord blood was stimulated with PHA, cellular proliferative response did not differ between infants with and without AD (11.3 ± 0.5 cpm vs 11.3 ± 0.9 cpm, P = 0.525) (Table 4). However, the ratio of cellular proliferative response of PHA-stimulated to -unstimulated cells was significantly higher in infants with than without AD (4.5 ± 0.9 cpm vs 4.1 ± 1.1 cpm, P = 0.048) (Fig. 1).
When we compared the levels of cytokines in the supernatants of PHA-stimulated cells of infants with and without AD (Table 4), we found no differences in the levels of IL-13 (P = 0.468) and IFN-γ (P = 0.231). In addition, the IFN-γ/IL-13 ratio did not differ between groups after adjustment for gender, maternal age at parity, gestational age, mode of delivery, maternal education level, season of birth and parental history of AD (P = 0.955).
We report the results of a prospective birth cohort study in Korea of risk factors for allergic diseases, especially AD, in children, and the immunologic markers for AD in their cord blood. We found that gender, mode of delivery and parental history of AD were important independent risk factors for diagnosis of AD at 12 months. We also found that CBMC proliferation in response to PHA stimulation may be a predictor of AD during infancy. However, there were no significant differences in IFN-γ and IL-13 levels following PHA stimulation of cord blood cells in infants with and without AD.
Several studies have reported that a maternal, but not a paternal, history of asthma or allergic disease is associated with the development of allergic diseases in infants (11, 16). In contrast, we found that both maternal and paternal histories of AD were associated with the development of AD in their offspring, suggesting that genetic influences may affect the early development of AD.
Several studies have reported that season of birth affected cord blood IgE level, although peak IgE levels differed among these studies (11, 16). We found, however, that season of birth did not differ between infants with and without AD at 12 months of age.
We also found that delivery by cesarean section was an independent risk factor compared with vaginal delivery for the development of AD at 12 months of age. Although several studies have reported an association between allergic diseases and delivery by cesarean section (17), others have not (18, 19). According to the hygiene hypothesis, unhygienic exposure decreases the risk of allergic disorders. Vaginally delivered children acquire their intestinal flora through the mother's vaginal tract; these flora reflect the mother's intestinal flora, mainly lactobacilli and bifidobacteria. In contrast, children born by cesarean section acquire their intestinal bacteria from skin contact and environmental surfaces. A recent meta-analysis reported that delivery by cesarean section increases the subsequent risk of asthma in children by about 20% (20).
Many studies have suggested that cord blood factors are associated with the development of allergic diseases. For example, children with high cord blood IgE levels have been reported to have a five-fold greater risk for developing asthma by 11 yr of age (21), although other studies failed to find such association (22). In contrast, family history of atopy was reported to be a better predictor of atopy than cord blood IgE (22). These discrepancies may be due to several factors, such as the cut-off level of cord blood IgE level, the duration of follow-up, ethnicity, outcome diseases, and populations (i.e. high-risk group vs the general population). We found that eosinophil counts, WBC counts, and total IgE levels in cord blood were similar in infants with and without AD. Since our group of infants was from the general population, our findings may reflect the actual condition of Korean infants. Our findings, however, require confirmation by larger study groups with longer follow-up duration.
The ability of proliferative response of cord blood cells to predict the development of allergic diseases is unclear, with some reports showing that proliferative response was not useful in predicting the outcome of allergic diseases (23, 24). In addition, immunoproliferative responses at 1 yr were reported to be unrelated to maternal mite allergen exposure during pregnancy, but were associated with increasing Der p 1 exposure at 1 yr (25). These findings suggest that infants early in life, but not in utero, become sensitized to inhaled allergens. In contrast, recent reports have shown that CBMCs obtained during gestation can proliferate after stimulation with various nutritive and inhaled allergens (26), as well as other types of allergens (27, 28). We found that CBMCs from infants with AD proliferated in response to PHA, but not OVA, stimulation, suggesting that potent cellular proliferation to nonspecific stimulation at birth may be associated with the development of allergic diseases, despite an absence of specific sensitization.
Neonatal T cells have been reported to preferentially show a Th2-dominant cytokine pattern and reduced Th1 cytokine production (29), even if they are pluripotent cells with the ability to produce both Th1 and Th2 cytokines (30). Changes in cytokine production may be associated with the subsequent development of allergic diseases. CD4+IL-13+ lymphocytes from CBMCs derived from atopic mothers were found to be associated with wheezing during the first year of life (10). Moreover, enhanced IL-13 levels at birth were associated with the subsequent development of atopic symptoms at 3 yr of age (23). In addition, in previous studies, IFN-γ release was significantly reduced in children with subsequent development of atopic disorders (15), whereas others, including our group, reported no association between IFN-γ response and allergy or sensitization (31). This may be due to differences among the recruited groups. Most of these studies included high-risk neonates with atopic parents, whereas our study group was recruited from the general population. Differences may also be due to methodological differences in cell culture technique, differences in stimulated allergens, and definitions of outcome.
IL-13 production has been related to allergic diseases, with IL-13 and IFN-γ production being markers of neonatal CD4+ T cell differentiation (32). In addition, IL-13 production has been correlated with the development of asthma and AD by the age of 1 yr (32), and with atopic symptoms at age 3 yr (22), suggesting that IL-13 may be an early marker for atopic disease in neonates who develop AD. We found, however, that levels of IL-13 production in response to PHA stimulation were similar in infants with and without AD. Since the development of allergic diseases is associated with a balance between Th1 and Th2 responses, increased IL-13 production may not independently affect the development of AD. Moreover, the IFN-γ/IL-13 was similar in infants with and without AD, suggesting that cytokine production in cord blood may not predict the development of AD during infancy.
The main outcome of this study was diagnosis of AD by pediatric allergy specialists. Although most other studies have used questionnaires completed by parents to define allergic diseases, we used a diagnosis by pediatric allergy specialists. Although the latter may be more accurate, the small number of patients can be limitation of our study. Allergic diseases show various phenotypes, so accurate definitions and diagnoses are very important.
The fetal period and early childhood are critical periods for detecting the onset of allergic diseases. Thus, interventions during this time may be effective in preventing allergic diseases. In conclusion, we found that gender, mode of delivery and parental history of AD had an effect on the development of AD at 12 months. A high cellular proliferative response of cord blood to PHA, rather than cytokine production in response to PHA, may be associated with infant development of AD at 12 months. Therefore, evaluating environmental factors and cellular status of cord blood may predict the development of AD in early infancy and these can be applied to prevent the development of allergic diseases.
Figures and Tables
Fig. 1
Cellular proliferative responses to ovalbumin (OVA) and phytohemagglutinin (PHA) stimulation in infants with and without atopic dermatitis at 12 months. Ratio of cellular proliferative responses in the presence and absence of (A) OVA and (B) PHA. The median values are presented and differences between the groups were reported as P values.

Table 3
Association between parental history of allergic diseases and diagnosis of atopic dermatitis at 12 months

Table 4
Cellular composition and cytokine levels of cord blood of infants with and without atopic dermatitis at 12 months
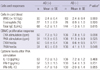
Data are all log-transformed. *Adjusted for gender, maternal age at parity, gestational age, mode of delivery, parental history of atopic dermatitis, maternal education level, and season of birth. AD, atopic dermatitis; WBC, white blood cell; CBMC, cord blood mononuclear cell; OVA, ovalbumin; PHA, phytohaemagglutinin.
ACKNOWLEDGMENTS
We thank Prof. Jung Yeon Shim (Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea), Prof. Woo Kyung Kim (Department of Pediatrics, Inje University Seoul Paik Hospital, Seoul, Korea), Prof. So-Yeon Lee (Department of Pediatrics, Hallym Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea), Prof. Soo Young Lee (Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea), Prof. Dae Jin Song (Department of Pediatrics, Korea University, Seoul, Korea), Prof. Gwang Cheon Jang (Department of Pediatrics, National Health Insurance Corporation Ilsan Hospital, Ilsan, Korea), Prof. Yee Jin Shin (Department of Psychiatry, College of Medicine Yonsei University, Seoul, Korea), Prof. Pil Ryang Lee (Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea) and Prof. Ho Kim (Graduate School of Public Health, Seoul National University, Seoul, Korea) for their brilliant and enthusiastic work to concrete and consult this cohort study. We also thank Mrs. Mi-Jin Kang and Mrs. Hee-Sook Kim (Asan Institute for Life Science, University of Ulsan College of Medicine, Seoul, Korea) for their excellent technical assistance and Mrs. Kyung-shin Lee and Ms Hae-Ri Lee (Asan Institute for Life Science, University of Ulsan College of Medicine, Seoul, Korea) for the valuable help with the statistical analyses. Prof. Cheol Min Lee (Institute of Environmental and Industrial Medicine, Hanyang University, Seoul, Korea) performed the environment measurement and Prof. Se-Young Oh (Department of Food and Nutrition, Kyung Hee University, Seoul, Korea) investigated the dietary intake of the enrolled subjects. We thank all the workers and families who spared no effort on COCOA study.
References
1. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006. 355:2226–2235.
2. Kwon JW, Kim BJ, Song Y, Seo JH, Kim TH, Yu J, Kim HB, Lee SY, Kim WK, Kim KW, et al. Changes in the prevalence of childhood asthma in Seoul from 1995 to 2008 and its risk factors. Allergy Asthma Immunol Res. 2011. 3:27–33.
3. Chung Y, Kwon JH, Kim J, Han Y, Lee SI, Ahn K. Retrospective analysis of the natural history of atopic dermatitis occurring in the first year of life in Korean children. J Korean Med Sci. 2012. 27:723–728.
4. Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, Lee SI. Prevalence of atopic dermatitis in Korea: analysis by using national statistics. J Korean Med Sci. 2012. 27:681–685.
5. Chung EK, Miller RL, Wilson MT, McGeady SJ, Culhane JF. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. Arch Dis Child Fetal Neonatal Ed. 2007. 92:F68–F73.
6. Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002. 32:43–50.
7. Moore MM, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Camargo CA Jr, Gold DR, Weiss ST, Gillman MW. Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics. 2004. 113(3 Pt 1):468–474.
8. Boyle RJ, Tang ML. Can allergic diseases be prevented prenatally? Allergy. 2006. 61:1423–1431.
9. Allam JP, Zivanovic O, Berg C, Gembruch U, Bieber T, Novak N. In search for predictive factors for atopy in human cord blood. Allergy. 2005. 60:743–750.
10. Spinozzi F, Agea E, Russano A, Bistoni O, Minelli L, Bologni D, Bertotto A, de Benedictis FM. CD4+IL13+ T lymphocytes at birth and the development of wheezing and/or asthma during the 1st year of life. Int Arch Allergy Immunol. 2001. 124:497–501.
11. Kaan A, Dimich-Ward H, Manfreda J, Becker A, Watson W, Ferquson A, Chan H, Chan-Yeung M. Cord blood IgE: its determinants and prediction of development of asthma and other allergic disorders at 12 months. Ann Allergy Asthma Immunol. 2000. 84:37–42.
12. Ferguson A, Dimich-Ward H, Becker A, Watson W, DyBuncio A, Carlsten C, Chan-Yeung M. Elevated cord blood IgE is associated with recurrent wheeze and atopy at 7 yr in a high risk cohort. Pediatr Allergy Immunol. 2009. 20:710–713.
13. Piastra M, Stabile A, Fioravanti G, Castagnola M, Pani G, Ria F. Cord blood mononuclear cell responsiveness to beta-lactoglobulin: T cell activity in 'atopy-prone' and 'non-atopy-prone' newborns. Int Arch Allergy Immunol. 1994. 104:358–365.
14. Piccinni MP, Mecacci F, Shampognaro S, Manetti R, Parronchi P, Maggi E, Romagnani S. Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol. 1993. 102:301–303.
15. Kondo N, Kobayashi Y, Shinoda S, Takenaka R, Teramoto T, Kaneko H, Fukao T, Matsui E, Kasahara K, Yokoyama Y. Reduced interferon gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders-6-year follow-up study. Clin Exp Allergy. 1998. 28:1340–1344.
16. Bjerke T, Hedegaard M, Henriksen TB, Nielsen BW, Schiøtz PO. Several genetic and environmental factors influence cord blood IgE concentration. Pediatr Allergy Immunol. 1994. 5:88–94.
17. Tollanes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr. 2008. 153:112–116.
18. Park YH, Kim KW, Choi BS, Jee HM, Sohn MH, Kim KE. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol Res. 2010. 2:28–33.
19. Sugiyama M, Arakawa H, Ozawa K, Mizuno T, Mochizuki H, Tokuyama K, Morikawa A. Early-life risk factors for occurrence of atopic dermatitis during the first year. Pediatrics. 2007. 119:e716–e723.
20. Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008. 38:629–633.
21. Croner S, Kjellman NI M. Development of atopic disease in relation to family history and cord blood IgE levels. Eleven year follow up in 1654 children. Pediatr Allergy Immunol. 1990. 1:14–20.
22. Hansen LG, Host A, Halken S, Holmskov A, Husby S, Lassen LB, Storm K, Osterballe O. Cord blood IgE II. Prediction of atopic disease. A follow-up at the age of 18 months. Allergy. 1992. 47:397–403.
23. Lange J, Ngoumou G, Berkenheide S, Moseler M, Mattes J, Kuehr J, Kopp MV. High interleukin-13 production by phytohaemagglutinin- and Der p 1-stimulated cord blood mononuclear cells is associated with the subsequent development of atopic dermatitis at the age of 3 years. Clin Exp Allergy. 2003. 33:1537–1543.
24. Chan-Yeung M, Ferguson A, Chan H, Dimich-Ward H, Watson W, Manfreda J, Becker A. Umbilical cord blood mononuclear cell proliferative response to house dust mite does not predict the development of allergic rhinitis and asthma. J Allergy Clin Immunol. 1999. 104:317–321.
25. Smillie FI, Elderfield AJ, Patel F, Cain G, Tavenier G, Brutsche M, Craven M, Custovic A, Woodcock A. Lymphoproliferative responses in cord blood and at one year: no evidence for the effect of in utero exposure to dust mite allergens. Clin Exp Allergy. 2001. 31:1194–1204.
26. Szepfalusi Z, Pichler J, Elsasser S, van Duren K, Ebner C, Bernaschek G, Urbanek R. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J Allergy Clin Immunol. 2000. 106:530–536.
27. Kondo N, Kobayashi Y, Shinoda S, Kasahara K, Kameyama T, Iwasa S, Orii T. Cord blood lymphocyte responses to food antigens for the prediction of allergic disorders. Arch Dis Child. 1992. 67:1003–1007.
28. Miles EA, Warner JA, Jones AC, Colwell BM, Bryant TN, Warner JO. Peripheral blood mononuclear cell proliferative responses in the first year of life in babies born to allergic parents. Clin Exp Allergy. 1996. 26:780–788.
29. Yabuhara A, Macaubas C, Prescott SL, Venaille TJ, Holt BJ, Habre W, Sly PD, Holt PG. TH2-polarized immunological memory to inhalant allergens in atopics is established during infancy and early childhood. Clin Exp Allergy. 1997. 27:1261–1269.
30. Kopp MV, Zehle C, Pichler J, Szépfalusi Z, Moseler M, Deichmann K, Forster J, Kuehr J. Allergen specific T-cell reactivity in cord blood: influence of maternal cytokine production. Clin Exp Allergy. 2001. 31:1536–1543.
31. Martinez FD, Stern DA, Wright AL, Holberg CJ, Taussig LM, Halonen M. Association of interleukin-2 and interferon-gamma production by blood mononuclear cells in infancy with parental allergy skin tests and with subsequent development of atopy. J Allergy Clin Immunol. 1995. 96:652–660.
32. Ohshima Y, Yasutomi M, Omata N, Yamada A, Fujisawa K, Kasuga K, Hiraoka M, Mayumi M. Dysregulation of IL-13 production by cord blood CD4+ T cells in associated with the subsequent development of atopic disease in infants. Pediatr Res. 2002. 51:195–200.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download