Abstract
The most promising treatment for stress urinary incontinence can be a cell therapy. We suggest human amniotic fluid stem cells (hAFSCs) as an alternative cell source. We established the optimum in vitro protocol for the differentiation from hAFSCs into muscle progenitors. These progenitors were transplanted into the injured urethral sphincter and their therapeutic effect was analyzed. For the development of an efficient differentiation system in vitro, we examined a commercial medium, co-culture and conditioned medium (CM) systems. After being treated with CM, hAFSCs were effectively developed into a muscle lineage. The progenitors were integrated into the host urethral sphincter and the host cell differentiation was stimulated in vivo. Urodynamic analysis showed significant increase of leak point pressure and closing pressure. Immunohistochemistry revealed the regeneration of circular muscle mass with normal appearance. Molecular analysis observed the expression of a larger number of target markers. In the immunogenicity analysis, the progenitor group had a scant CD8 lymphocyte. In tumorigenicity, the progenitors showed no teratoma formation. These results suggest that hAFSCs can effectively be differentiated into muscle progenitors in CM and that the hAFSC-derived muscle progenitors are an accessible cell source for the regeneration of injured urethral sphincter.
Stress urinary incontinence (SUI) is the involuntary leakage of urine that can occur during coughing, laughing, sneezing, or jogging (1). SUI can be caused by a childbirth, weight gain, or other conditions that stretch the pelvic floor muscles (1). Treatment modalities of SUI include behavioral techniques, physical therapy, medications, medical devices, interventional therapies and surgical treatments (2). Unfortunately, these treatments are not always effective or may have associations with adverse events (3). Recently, muscle progenitor cells were known to have clinically relevant therapeutic potential for the treatment of SUI (4). The benefits of the progenitor cell therapy are; safety, long-term survival and potential effectiveness (5). The progenitor cells can be induced from immune privileged stem cells obtained from auto- (5) or allo-genic (4) biopsy. The isolated cells are multiplied for 4-6 weeks and injected into the urethral sphincter muscles that control urination and prevent leakage (6). But performing a biopsy procedure to obtain progenitor cell can cause complications in patients such as pain, swelling or bleeding.
As a noninvasive stem cell source, human amniotic fluid stem cell (hAFSC) is readily available from routine clinical amniocenteses at the second trimester. Both embryonic and adult stem cell markers can be expressed from these cells retaining long telomeres and a normal karyotype for more than 250 doubling times, and can be differentiated into any of the three germ layer cells (7). These characteristics make hAFSCs a potential source of stem cells for cell-based therapeutic strategies.
Various approaches to the directed and efficient differentiation of hAFSCs into muscle progenitors have been reported (8). These methods include co-culture (9), growth factors (10), chemicals (8) and/or mechanical force (11), but these techniques have limitations that their usages result in low differentiation yields, have high inconsistency in differentiation efficiency and require expensive reagents. Until present, no optimized pre-differentiation system of hAFSC for the management of urinary incontinence has been demonstrated. Therefore, we examined whether hAFSCs can be effectively induced to muscle progenitor cells in which lineage-specific genes and proteins are expressed. We then conducted an in vivo experiment to determine whether hAFSCs-derived muscle progenitors have a potential for the regeneration of injured urethral sphincter.
This study was approved by the Ethics Committee of Kyungpook National University School of Medicine. Samples of 10 mL amniotic fluid were obtained with informed consent from 4 women who were undergoing amniocentesis for prenatal diagnosis at 15-19 weeks of pregnancy. The amniotic fluid was centrifuged and the pellets were resuspended in the Chang Medium (α-MEM, 15% embryonic stem cell-fetal bovine serum [Gibco-Invitrogen, Grand island, NY, USA] with 18% Chang B and 2% Chang C [Irvine Scientific, Irvine, CA, USA]) and placed on a petri dish. Non-adherent cells were discarded at 1 week. Adherent cells were passaged for expansion when they reached 80% confluence. The culture medium was replaced every 3 days.
At passage 3, the cultured cells were characterized with mesenchymal (SSEA4, CD44, CD73, CD 90, and CD105) and hematopoietic (CD45) stem cell markers using Fluorescent Activated Cell Sorting (FACS) (BD Biosciences, San Jose, CA, USA). To obtain genetically homogenous stem cell population, cells were sorted twice using C-KIT antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) with magnetic activated cell sorting system (MACS, Miltenyi Biotec, Germany). The case with highest C-KIT (+) population was selected subsequently for the study. The population from selected case was then immuno-selected twice using an antibody against c-kit (Santa Cruz Biotechnology).
To establish the optimum condition for pre-differentiation of hAFSCs into myogenic lineage, commercial medium, co-culture with primary cells and conditioned medium (CM) were compared. Commercial differentiation media were purchased from Gibco-Invitrogen (Medium 231 with smooth muscle growth supplement). Co-culture was performed using a 6-well insert (BD Biosciences) in which hAFSCs were seeded on a petri dish and primary skeletal muscle cells were loaded on the well insert. The primary cells were provided by Dr. BS Kim (Kyungpook National University Hospital, Daegu, Korea). The CM was collected every 3 days from the primary cell culture medium. For pre-differentiation, hAFSCs were treated for 7 days in the medium. To determine the genotypic conversion of hAFSCs into myogenic lineage cells, real-time PCR and immunocytochemical (ICC) staining were performed.
Total RNA was isolated from Trizol Reagent and cDNA was prepared from 2 µg of total RNA using the SuperScript first-strand synthesis system (Gibco-Invitrogen). Amplification was performed with triplicate assays and reactions were instigated in a 96-well plate in 20 µL of reaction agent containing SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), 2 pM of each forward and reverse primer, and a cDNA template corresponding to 400 pg of total RNA. The primer sequences of the candidate genes and β-ACTIN (internal control) are shown in Table 1. SYBR Green PCR conditions were applied at 95℃ for 10 min followed by 45 cycles of 95℃ for 10 sec, 58℃ for 50 sec, and 72℃ for 20 sec. For the analysis of the relative changes in gene expression, 2-ΔΔCt method was used.
After the differentiation, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature and permeabilized with 0.1% Triton X-100 (Sigma, St. Louis, MO, USA) for 20 min. These cells were then incubated overnight at 4℃ with primary antibodies against MYOD, DESMIN for myogenic differentiation (Table 2). After three washes with PBS, FITC-conjugated secondary antibodies diluted to 1:500 were added and incubated at 37℃ for 1 hr. Then, 1 µg/mL of 4',6-diamino-2-phenylindole was used to stain the cell nuclei. The cells were visualized and photographed using fluorescence microscopy.
The growth rate of the muscle progenitor cells in CM was evaluated using Cell Counting Kit-8 (CCK-8, Dojindo, Japan). To describe the process briefly, the cells were induced to muscle progenitors in 96-well plates for 14 days with proliferation medium serving as the control, and the muscle progenitor cells were prepared for experiment groups. At each time point, 10 µL of CCK-8 solution was added to each well and the 96-well plate was continuously incubated at 37℃ for 1 hr, then the optical density (OD) value on a microplate reader was read at wavelength 450 nm to determine the cell growth rate.
All experimental protocols were approved by the Animal Ethics Committee, Kyungpook National University School of Medicine. A total of 40 female ICR mice weighing 20-25 g each were obtained from Hyochang Science (Daegu, Korea). Animals were prepared for an aseptic surgery under general anesthesia (isoflurane). A lower midline abdominal incision was made and the bladder and the urethra were exposed. The pudendal nerve on each side was identified and transected with microsurgical scissors under microscopic magnification (n = 30). The laparotomy site was closed in layers with absorbable 4-0 vicryl sutures. Another 15 mice underwent a sham operation (lower midline incision and closure) and served as the normal control.
One week after the generation of the incompetent urethral sphincter model, animals were anesthetized with isoflurane and their bladder and urethra were exposed by a lower abdominal incision. Approximately 0.5 × 106 cells in 3 µL of suspension were injected into the external sphincter of each mouse. Four groups were established: sham-operation group (Ctrl [+]), pudendal neurectomy without cell injection group (Ctrl [-]), and pudendal neurectomy with hAFSC or muscle progenitor cell injection group (each n = 10). The animals were sacrificed 2 and 4 weeks after the transplantation.
Two and 4 weeks after the cell injection, mice were placed under general anesthesia with ether to avoid muscle relaxation and LPP and CP were measured using the vertical tilt/intravesical pressure clamp model of SUI (12). Before taking the measurements, the spinal cord was transected at the T9-T10 level to eliminate the reflex bladder activity in response to the increased intravesical pressure. Under general anesthesia, a transvesical catheter with a fire-flared tip (PE-25) was inserted into the dome of the bladder. The mice were then mounted on a tilt table and placed in the vertical position. The intravesical pressure was increased in 1 to 3 cmH2O steps from 0 cmH2O upward until the visual identification of the leak point pressure was made. The pressure at this leak point was referred to as the LPP. The intravesical pressure was then decreased in 1 to 3 cmH2O steps downward until the leak ceased. The pressure at this leak cessation point was referred to as the CP. The averages of three consecutive LPP and CP measurements were taken as the data points for each animal.
Animals were sacrificed after the LPP and CP measurements. The bladder-urethra complex was then removed en bloc. Tissue specimens were fixed in 10% buffered formalin, processed, and cut into 4-µm-thick slices for staining with hematoxylin & eosin (H&E). The degree of urethral sphincter recovery from injury was assessed using histological and immunohistochemical (IHC) stain with MYOD and Sooth muscle (SM) actin antibodies. In the real-time PCR analysis, myogenic related genes (Pax7, Myf5, MyoD, Myogenin) expression within the host urethral sphincter was analyzed using mouse PCR primers.
The presence, migration and duration of injected human cells in situ were analyzed with IHC using anti-human nuclear antibody (Cell Signaling Technology, Danvers, MA, USA) at 1 and 2 weeks after the injection. The immunogenicity of muscle progenitor cells was evaluated with the expression of HLA-DR on their surface using FACS. To evaluate in vivo immunosuppression effect, tissues were retrieved 2 weeks after the injection and IHC staining with cytotoxic T cell marker (CD8, BD Pharmigen, San Jose, CA, USA) was performed. Human fibroblasts were used as the positive control and sham operated mice were used as the negative control. For tumorigenicity analysis, 1 × 106 muscle progenitor cells were transplanted into the subcapsular space of the kidney (each group, n = 3). Four weeks later, animals were sacrificed and the kidneys were harvested for histologic confirmation.
Our main outcome was a comparison of the differences between LPP and CP between the muscle progenitor cells injection group and the control group in the SUI animal model. We collected data from 5 animals in each group 2 and 4 weeks after the injection. The results were also compared with those of the normal control group (n = 5, at each time point). All data were presented as means±SD. Statistical analysis was undertaken with Student's t-test or nonparametric analysis. A P value of less than 0.05 was considered statistically significant.
After three passages of culture, human amniotic fluid-derived cells became morphologically homogeneous and exhibited a fibroblast-like cell type. The mean expression (%) of mesenchymal markers evaluated using flow cytometry for CD44, CD73, CD90, CD105, SSEA-4, and HLA-ABC was 98.58±1.02, 97.11±2.48, 96.74±3.29, 85.67±9.59, 14.31±4.75, and 64.02±19.20, respectively. The mean expression (%) of CD45 hematopoietic marker was 2.16±1.02 and HLA-DR immune response-related marker was 1.14±0.98. Human amniotic fluid-derived cells from 4 cases were sorted with c-kit stem cell marker to obtain genetically homogeneous cell populations. Positive cell population of each case was 5.00, 3.49, 3.57, and 0.60, respectively. The case with highest positive population was selected for the second c-kit sorting which turned out to be the case #1 with 98.4% positive cell population. Study progressed to the next experimentation step with this cell population.
Real-time PCR revealed significant changes in lineage-specific genes expression during differentiation. The undifferentiated hAFSCs showed higher expression of stem markers (SMAD2, OCT4) than myogenic lineage markers (PAX7, MYOD) (P < 0.001) (Fig. 1). During myogenesis, cells showed more elongated morphology in CM than in other media. The detection rates of the early expressed genes, PAX7, MYF5, and MYOD mRNA, were high after 3 days of induction. Their expression decreased from day 7. CM showed relatively enhanced myogenic genes expression compared with the control and co-culture systems. In ICC analysis, CM showed enhanced MYOD expression. The expression level of the middle development marker, DESMIN, was similar among 3 media (Fig. 2A).
The OD value of control cells (in proliferation medium) at each time point was 0.88±0.02, 1.40±0.09, 1.12±0.13, and 1.38±0.08, respectively. The same of the muscle progenitors was 0.78±0.05, 1.24±0.07, 0.42±0.02, and 0.41±0.07, respectively. The proliferation rates of the control vs muscle progenitors at day 1 and 3 were similar (P = 0.085). While the growth rate of the muscle progenitor cells significantly decreased from day 7; that of the differentiation medium-treated cells was notably slow. This inhibition trend continued until day 14 (P < 0.001) (Fig. 2B).
LPP and CP were measured 2 and 4 weeks after the injection (Fig. 3A). The mean LPP of the Ctrl (-), hAFSC, muscle progenitor and Ctrl (+) group at week 2 was 12.78±2.89, 18.06±2.60, 36.96±1.30, and 36.13±2.53 cmH2O, respectively. At week 4, the mean LPP of each group was 15.24±1.87, 23.90±1.85, 38.43±0.51, and 36.54±1.67 cmH2O, respectively. The LPP of muscle progenitor cell injection group was significantly higher than that of the Ctrl (-) and hAFSC groups (P = 0.010 at week 2 and P = 0.007 at week 4). The mean CP of the Ctrl (-), hAFSC, muscle progenitor and Ctrl (+) groups at week 2 was 10.98±2.46, 12.42±1.50, 21.84±1.13, and 22.68±3.03 cmH2O, respectively. At week 4, the mean CP of each group was 8.35±1.74, 16.64±3.09, 28.38±1.41, and 25.73±2.06 cmH2O, respectively. The mean CP of the muscle progenitor group was significantly higher than that of the Ctrl (-) and hAFSC groups (P = 0.015 at week 2 and P = 0.007 at week 4) (Fig. 3A).
H&E staining revealed the histological advantages of muscle progenitor cell injection with normal-appearing circular muscle mass regeneration at the urethral sphincter at week 4. The muscle progenitor transplantation showed the enhanced regeneration compared with the hAFSC group. The Ctrl (-) group showed only scant muscles with atrophic sphincters. In the analysis of the IHC results, immunofluorescence signal intensities of the Ctrl (+) and Ctrl (-) tissues were used as the control. The muscle progenitor transplantation significantly stimulated the expressions of muscle-related markers. The above results proved the benefit of the muscle progenitor transplantation using the urethral sphincter repair process (Fig. 3B).
To determine the genetic effects of the progenitors on the differentiation of the host tissue, we performed the detection of the mRNA levels with differentiation markers for myogenesis using real-time PCR. The myogenic genes expressions related with early (PAX7, MYF5, MyoD) and mid to late (MYOGENIN) differentiations well matched with time. Compared with the Ctrl (-), the muscle progenitor transplantation group showed the relatively high myogenic genes expressions for the time (P = 0.005). These results suggest that muscle progenitor treatment can promote host cell differentiation (Fig. 3C).
Using the human nuclear specific antibody, we performed the detection of the injected human cells. The stains nuclei of the human cells were given a diffuse staining pattern. The urethral sphincters treated with the muscle progenitors showed positive staining at week 2 but these signals were not detected at week 4. Transplanted cells were evenly distributed and were sometimes grouped in small clusters (Fig. 3D). The immunologic analysis with IHC staining revealed significant differences between the cell injection groups and the Ctrl (+) group at week 2. The cell injection groups had a scant CD8 lymphocyte aggregations at the urethral sphincters. Their signal intensity was lower than that of the Ctrl (-) group, while the Ctrl (+) animals injected with human fibroblasts showed significant CD8 lymphocyte accumulations (Fig. 3D). On in vivo tumorigenicity analysis, there was no teratoma formations in tissues retrieved 4 weeks after the renal subcapsular injection of pre-differentiated hAFSCs (Fig. 3D).
In this study, we demonstrated that hAFSCs have many multipotent mesenchymal stem cell properties with the expressions of CD44, CD73, CD90, CD105, and SSEA4 and the absence of CD45 expression. The double sorted hAFSCs (with c-kit) produced the expression of 98.4% positive homogeneous cell population. In real-time PCR analysis, the c-kit positive cells showed significantly high expression of stem cell markers. These findings may indicate that hAFSCs closely resemble the multipotent mesenchymal stem cells. hAFSCs also showed low expression rate of myogenic cell markers, which led to a hypothesis that hAFSCs are less differentiated but can be easily differentiated into myogenic lineage cells.
To find out the effective cell therapy for SUI, we adopted a novel strategy to induce pre-differentiation of hAFSCs into myogenic progenitor cells. We performed tests for the introduction of a pre-treatment stage to the study using commercial medium, co-culture or CM system. Our results showed that the CM system inhibits the growth of hAFSCs and effectively facilitated the differentiation into myogenic cell types compared with other systems, but the responsible mechanism needs to be found. The myogenic progenitor cells displayed the morphology similar to that of the lineage and expressed myogenic mRNA (PAX7, MYF5, MYOD). The expressions of MYOD and DESMIN also were confirmed at the protein level in the ICC analysis.
Urodynamic studies on an animal model was performed for the evaluation of LPP and CP. Four weeks after the cell transplantation, we found significant increases of LPP and CP in the muscle progenitor injection group, and the increase level was similar to the normal while the hAFSCs injection group showed low therapeutic effects. The increases of LPP and CP after the muscle progenitor injection were caused by the improvements of external urethral sphincter muscles, which were proved by the results of IHC and real-time PCR. Therefore, we propose that muscle progenitor injection into the injured urethra can improve urethral sphincter function, probably because the pre-differentiated hAFSCs stimulate the host muscle cells.
In the histological and IHC staining, the injected muscle progenitor cells could accelerate sphincter muscle regeneration through the fusion with the host cells and the activate local muscle progenitor cells. The muscle progenitors showed increased muscle bundles in the urethral sphincter and the significant positive MyoD and SM actin markers, which may lead to the enhanced contractility and decreased SUI symptoms in an animal model. In addition, muscle progenitors did not cause malformation in terms of pathology. The Ctrl (-) group showed decreased expressions of these markers. In real-time PCR analysis, the muscle progenitor group showed a significant correlation between gene and protein expressions. These results suggest that the muscle progenitor has a therapeutic property and the function of improving the damaged sphincter.
It has been reported that undifferentiated hAFSCs can be integrated into the damaged tissues and differentiated into the target cell lineage in rodent models (13, 14), and that the differentiated cells could survive for more than 3 months (14). However, the fate of the hAFSCs-derived muscle progenitor after the in vivo transplantation in urethral sphincter has yet to be determined. We found that the hAFSCs-derived muscle progenitor could survive for 2 weeks and be integrated towards the host muscle region, which was proved by IHC analyses using human nuclei-specific antibodies. These results indicate that the hAFSCs-derived muscle progenitor have a short-lived potential to survive, but further stimulate the ability of the host cell to the differentiation into the functional myogenic cells.
The FACS study suggested that hAFSCs caused expression of HLA-DR less than that of isotope control, and that the injection of hAFSCs-derived muscle progenitor grafts into the injured urethral sphincter did not stimulate CD8+ T cell infiltration. These findings suggest that the hAFSCs-derived muscle progenitor cells have an immune tolerance and/or immunesuppression effects. The tumorigenicity analysis using renal subcapsule revealed that these cells did not produce teratoma for 4 weeks, because the committed progenitors are usually postmitotic (15). This means that the hAFSCs-derived muscle progenitors might be safe in terms of tumorigenicity when applied in a cell therapy.
The limitations of this study were the lack of a precise mechanism for a paracrine effect, and the inability to track the metabolism or fate of the injected cells in vivo.
Conditioned medium proved to be an effective system for pre-differentiation of hAFSCs into muscle lineage progenitors. The injection of the hAFSCs-derived muscle progenitor cells into the denervated external urethral sphincter increased myogenic-related markers expression and urodynamic function. The muscle progenitor cells showed therapeutic effect on SUI. It is suggested that the muscle progenitors are fused with the host tissue, gradually differentiate into the muscle lineage cells, and contribute to the regeneration of the injured urethral sphincter.
Figures and Tables
 | Fig. 1Stem cell and myogenic lineage markers expression of undifferentiated hAFSCs. Undifferentiated hAFSCs produced significantly higher expression of stem cell markers compared to myogenic lineage cell markers. |
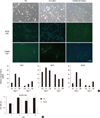 | Fig. 2Expression of myogenic markers in hAFSCs-derived muscle progenitor cells. (A) Representative immunocytochemical (at day 7) and real-time PCR analysis of the expression of muscle progenitor markers. Conditioned medium treated cells showed relatively elongated morphology and enhanced myogenic gene expression for PAX7 and MYOD compared with the control and co-culture systems. Ctrl, commercial medium; Co, co-culture; CM, conditioned medium. (B) The growth rate of hAFSCs-derived muscle progenitor cells in CM measured for 14 days. The growth rate of hAFSCs-derived muscle progenitor cells in CM was inhibited because the differentiating cells are usually postmitotic (*P < 0.001; †P < 0.001). Ctrl, undifferentiated hAFSCs; Muscle, hAFSCs-derived muscle progenitor cells. |
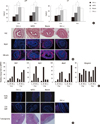 | Fig. 3Therapeutic property of muscle progenitor cells for urethral sphincter regeneration. In urodynamic study (A), leak point pressure (LPP) and closing pressure (CP) improved significantly in animals with pudendal neurectomy in the muscle progenitor group at week 2 and 4, compared with those in the hAFSC group (*P = 0.003, †P = 0.003, ‡P = 0.008, §P = 0.008). The representative H&E and IHC images (B) and real-time PCR (C) results of the muscle progenitor group exhibited the accelerated sphincter regeneration with myogenic lineage markers expression. The results of injected human cell detection, immune responses and a safety study in vivo (D) showed that hAFSCs-derived muscle progenitor cells showed their integration into the host tissue at week 2, scant CD8 lymphocyte aggregation in the urethral sphincter at week 2 and no teratoma formation under renal capsule at week 4. Ctrl (-), bilateral pudendal nerve transected group; hAFSC, bilateral pudendal nerve transected and hAFSC injected group; Muscle, bilateral pudendal nerve transected and hAFSCs-derived muscle progenitor cell injected group; Ctrl (+), sham operated group. |
References
1. Deng DY. Urinary incontinence in women. Med Clin North Am. 2011. 95:101–109.
2. Sassani P, Aboseif SR. Stress urinary incontinence in women. Curr Urol Rep. 2009. 10:333–337.
3. Madjar S, Sharma AK, Waltzer WC, Frischer Z, Secrest CL. Periurethral mass formations following bulking agent injection for the treatment of urinary incontinence. J Urol. 2006. 175:1408–1410.
4. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.
5. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.
6. Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev. 2012. 21:834–843.
7. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007. 25:100–106.
8. Gekas J, Walther G, Skuk D, Bujold E, Harvey I, Bertrand OF. In vitro and in vivo study of human amniotic fluid-derived stem cell differentiation into myogenic lineage. Clin Exp Med. 2010. 10:1–6.
9. Bollini S, Pozzobon M, Nobles M, Riegler J, Dong X, Piccoli M, Chiavegato A, Price AN, Ghionzoli M, Cheung KK, et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011. 7:364–380.
10. Yeh YC, Wei HJ, Lee WY, Yu CL, Chang Y, Hsu LW, Chung MF, Tsai MS, Hwang SM, Sung HW. Cellular cardiomyoplasty with human amniotic fluid stem cells: in vitro and in vivo studies. Tissue Eng Part A. 2010. 16:1925–1936.
11. Zhang XH, Zeng ZP, Li HZ, Zhou YR, Zhang J, Tong AL, Yan ZL. Expression of renin-angiotensin-aldosterone system in human adipose tissues. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006. 28:766–769.
12. Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, Chancellor MB. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004. 23:166–171.
13. Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008. 26:2902–2911.
14. Cipriani S, Bonini D, Marchina E, Balgkouranidou I, Caimi L, Grassi Zucconi G, Barlati S. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. 2007. 31:845–850.
15. Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010. 20:233–243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download