Abstract
Previous studies suggested that polymorphisms of proinflammatory cytokine genes are important host genetic factors in Helicobacter pylori infection. The present study evaluated whether IL-8-251 polymorphism affected H. pylori eradication rate and to investigate the effect of H. pylori eradication on angiogenesis and the inflammatory process according to the IL-8-251 polymorphism. A total of 250 H. pylori-positive patients treated by endoscopic resection of the gastric neoplasm were classified into 3 groups (134 H. pylori-eradicated group, 19 H. pylori-eradication failure group, and 97 H. pylori-infected group). H. pylori status, histology, and angiogenic factor levels were evaluated at baseline, 6 months, and 18 months. H. pylori eradication rate was 92.9% in AA genotype, 85.7% in AT genotype and 88.4% in TT genotype (P value = 0.731). Elevated IL-8 and matrix metalloproteinase-9 concentrations in H. pylori-infected gastric mucosa were reversible by successful eradication of H. pylori, independent of the IL-8-251 polymorphism. It is suggested that elevated IL-8 and MMP-9 concentrations in H. pylori-infected gastric mucosa are altered significantly after successful eradication and these conditions continue for 18 months. However, IL-8-251 polymorphism does not affect H. pylori eradication rate and the sequential changes of related angiogenic factors after H. pylori eradication in Koreans.
Helicobacter pylori infection plays a major role in gastric carcinogenesis, commencing with chronic gastritis and leading to a sequence of atrophic gastritis, metaplasia, dysplasia, and subsequently, cancer (1, 2). Host genetic factors, bacterial virulence, and environmental factors are associated with gastric carcinogenesis in H. pylori-infected hosts. Recent reports have suggested that polymorphisms of proinflammatory cytokine genes are important host genetic factors in H. pylori infection (3-8).
Interleukin-8 (IL-8), a potent chemoattractant for neutrophils and lymphocytes, has been reported as a strong stimulator of angiogenesis in gastric adenocarcinoma (9-12). In Korea, the IL-8-251 A allele has been associated with higher IL-8 production, more severe inflammation, mucosal atrophy, and intestinal metaplasia when compared with the IL-8-251 TT genotype of H. pylori-infected patients. Recent studies suggested a possible association of the IL-8-251 A allele with angiogenesis and inflammation in gastric carcinogenesis in H. pylori-infected Koreans (13, 14). H. pylori infection induces several angiogenic factors and proteases, including vascular endothelial growth factor (VEGF), angiopoietin and matrix metalloproteinase-9 (MMP-9) (11, 15).
There has been no data to show the association of IL-8-251 polymorphism and H. pylori eradication in gastric neoplasm. The primary aim of this study was to evaluate whether IL-8-251 polymorphism affects H. pylori eradication rate. The secondary aim was to evaluate the effect of H. pylori eradication on angiogenesis and the inflammatory process in Koreans according to the IL-8 polymorphism, after endoscopic resection of gastric neoplasms.
From April 2005 to June 2008, patients who underwent endoscopic resection of gastric adenoma or early gastric cancer (EGC) at Seoul National University Hospital were prospectively enrolled and retrospectively reviewed. The presence of H. pylori infection was confirmed with rapid urease test and/or histology prior to endoscopic resection. Patients with a history of gastric surgery, H. pylori eradication therapy, or systemic disease requiring chronic medication were excluded. Patients who had used proton pump inhibitors (PPI), nonsteroidal anti-inflammatory drugs, or antibiotics within 4 weeks of enrollment were also excluded.
Patients received either H. pylori eradication treatment or were followed-up without eradication after endoscopic resection of gastric neoplasm. Patients in the eradication group received omeprazole 20 mg twice daily, amoxicillin 1,000 mg twice daily, and clarithromycin 500 mg twice daily for a week, whereas patients in the follow-up group received standard care without eradication of H. pylori.
Patients underwent an endoscopic follow-up examination at 3 months, 6 months, 12 months, and 18 months after endoscopic resection to evaluate gastric histology and angiogenic factor levels. During the follow-up period, biopsy samples were taken for evaluation of H. pylori status, histology, and angiogenic factors.
One piece of gastric mucosal tissue was taken from a non-lesional area of the antrum, and genomic DNA was isolated using a standard proteinase K digestion and phenol/chloroform extraction. The IL-8-251 A/T polymorphism was identified using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis, as previously described (16). For PCR-RFLP, the genomic region containing the IL-8-251 polymorphism was amplified with the forward (5'-TCA TCC ATG ATC TTG TTC TAA-3') and reverse (5'-GGA AAA CGC TGT AGG TCA GA-3') primers, using the PerkinElmer model 9600 (PerkinElmer Co., Norwalk, CT, USA), programmed at 95℃ for 5 min, 35 amplification cycles (95℃ for 30 sec, 54℃ for 1 min and 72℃ for 1 min), followed by 72℃ for 5 min. Following PCR, the MfeI (New England Biolabs Inc., Beverly, MA, USA) restriction enzyme was used, and the enzyme-digested products were applied to a 2% agarose-gel running electrophoresis system. IL-8-251 AA, AT and TT genotypes were determined according to the expected band patterns visualized on the agarose gel; the AA genotype showed 92 and 450 base-pair bands, whereas the TT genotype showed a 542 base-pair band, and the AT genotype showed 92, 450, and 542 base-pair bands (13, 16).
H. pylori infection was defined if results from either the rapid urease test (CLOtest; Kimberley-Clark, Draper, UT, USA) or modified Giemsa staining of the antral and/or body mucosa were positive. H. pylori eradication was defined if both results from follow-up biopsy were negative. Two pieces of gastric tissue, one from the non-lesional mucosa of the lesser curvature side of the antrum and the other from the midbody, were taken for histological evaluation using the updated Sydney system (17). The degree of H. pylori density, neutrophil infiltration, mononuclear cell infiltration, atrophy, and intestinal metaplasia were assessed and scored as 0 = none, 1 = mild, 2 = moderate, and 3 = marked. Specimens were examined in a blinded manner by a single pathologist.
Two pieces of mucosal tissue were obtained from the non-lesional gastric antrum during endoscopic examination. For analysis, the tissues were homogenized using a tissue homogenizer (Thomas scientific, Swedesboro, NJ, USA); supernatants were taken after centrifugation at 10,000 g for 10 min. The total protein amount was measured using a modified Lowry method. Commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MN, USA) were used according to manufacturer instructions for immunological quantification of IL-8, VEGF, MMP-9, and Ang-1. All assays on samples and controls were performed in duplicate, and the two results were averaged. Mucosal concentrations of cytokines were expressed as picogram or nanogram of cytokine per milligram of total protein (pg/mg or ng/mg protein).
Baseline characteristics were compared using the chi-square test and the Student's t-test for categorical and continuous variables, respectively. Following endoscopic resection, changes in histological scores and angiogenic factor levels were compared in relation to IL-8-251 genotypes (A carriers, including AA and AT vs TT) using the one-way ANOVA. A P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA).
Data were collected prospectively from consecutive patients who underwent endoscopic resection of the gastric neoplasm, and then retrospectively reviewed. Among a total of 250 H. pylori-positive patients treated by endoscopic resection, 153 patients (61.2%) received eradication therapy, and 97 patients (38.8%) were followed-up without eradication. H. pylori was successfully eradicated in 134 patients (eradication rate 87.6%). A total of 19 patients were classified as eradication failure group who were treated with H. pylori eradication treatment but had persistent H. pylori infection. Clinical and pathological findings were compared among the 3 groups (134 H. pylori-eradicated group, 19 H. pylori-eradication failure group, and 97 H. pylori-infected group). No significant differences were noted among the 3 groups in terms of mean age, gender ratio, gastric disease, and IL-8-251 polymorphism (Table 1).
Successful eradication rate of H. pylori was compared depending on the IL-8-251 A/T polymorphism. H. pylori eradication rate was 92.9% in AA genotype, 85.7% in AT genotype and 88.4% in TT genotype (P value = 0.731). A comparison between A carriers (H. pylori eradication rate 86.9%) and TT genotype also showed no significant difference (P value = 0.779).
Following endoscopic resection, changes in histological scores in the gastric antrum were compared in relation to IL-8-251 genotypes in the 3 groups, respectively (Fig. 1). Mean scores for neutrophil and monocyte infiltration, which represents acute and chronic inflammation, were significantly improved in the H. pylori-eradicated group, independent of IL-8-251 genotypes. There were no significant differences in mucosal atrophy and intestinal metaplasia in all 3 groups, independent of IL-8-251 genotypes.
During the follow-up period, changes in IL-8 concentrations and angiogenic factors were compared in relation to IL-8-251 genotypes in the 3 groups, respectively (Fig. 2). IL-8 and MMP-9 concentrations showed a significant reduction for 18 months follow-up period in the eradicated group, irrespective of IL-8-251 genotypes. However, IL-8 and MMP-9 levels in the H. pylori-infected group and H. pylori-eradication failure group showed no significant changes during the follow-up period. In contrast, VEGF levels showed a significant reduction in the H. pylori-infected group. Ang-1 levels showed no significant changes during the follow-up period in all 3 groups.
Angiogenic factor levels at 6 months and 18 months after endoscopic resection were compared between IL-8-251 A carriers and IL-8-251 TT genotypes. No significant difference was observed in all angiogenic factor levels between IL-8-251 A carrier and TT genotypes, irrespective of H. pylori eradication.
In Korea, gastric adenoma and EGC without concomitant lymph node metastasis are often treated with endoscopic resection. Because endoscopic resection removes only the tumor and surrounding mucosa, a metachronous gastric tumor can develop in the remaining gastric mucosa (18, 19). Previous studies to determine whether or not H. pylori eradication reduces preneoplastic lesions and the prevalence of gastric cancer have been inconclusive (20-22). Polymorphism of the IL-8 gene has recently been associated with important host genetic factors for gastric carcinogenesis (4, 13). Findings from a recent study indicated that H. pylori infection causally promoted host angiogenesis, which has been attributed to either augmented inflammation or enhanced carcinogenesis, and proton pump inhibitor could potentially be used for inhibition of H. pylori-provoked angiogenesis (23).
Previous studies suggested that the IL-8-251 A allele is associated with severe inflammation and angiogenesis in H. pylori-infected Koreans (13, 14). However, there has been no data to see the association of IL-8-251 polymorphism and H. pylori eradication in gastric neoplasm. Therefore we aimed to evaluate whether IL-8-251 polymorphism affects H. pylori eradication rate in the first place. There was no significant difference in the H. pylori eradication rate depending on the IL-8-251 A/T polymorphism. This result suggested that IL-8-251 polymorphism did not affect H. pylori eradication rate.
As expected, H. pylori eradication induced significant improvement in acute and chronic inflammation characterized by neutrophil and monocyte infiltration. However, these improvements in the inflammatory process did not lead to recovery of preexisting atrophy and intestinal metaplasia. In consideration of genetic polymorphisms, there was no significant difference in gastritis scores between the IL-8-251 A allele and the TT genotypes. Results from previous studies have suggested that the IL-8-251 A allele is associated with severe inflammation, atrophy, and intestinal metaplasia of gastric body mucosa in patients aged 49 or younger; however, these phenomena were not observed in the older age group (13). Because the mean age of patients was 60.5 yr in our study, this could explain the lack of an association and would be consistent with the previous results. Furthermore, results concerning the effects of H. pylori eradication on premalignant conditions such as atrophic gastritis and intestinal metaplasia, are conflicted (24-26). In this study, the duration of follow-up after endoscopic resection was not long enough to evaluate the effect of H. pylori eradication on atrophy and intestinal metaplasia.
H. pylori infection was recently reported to induce IL-8 and MMP-9 expression in gastric mucosa, and increment of these factors contributes to tissue damage in H. pylori-associated gastritis (11, 27). In this study, IL-8 and MMP-9 concentrations were significantly decreased in gastric mucosa following H. pylori eradication, and low concentrations were maintained during the follow-up period. This result could indicate that down-regulation of IL-8 and MMP-9 by eradication of H. pylori might inhibit gastric mucosal inflammation and angiogenesis which is essential to gastric carcinogenesis. In the present study, VEGF concentrations in the H. pylori-infected group were significantly decreased unlike IL-8 and MMP-9 concentrations. VEGF showed varied results in relation to H. pylori infection in previous studies. One study reported that H. pylori down-regulated VEGF receptors in vascular endothelial cells and this could explain our results (28). Findings from a recent study revealed significant correlation between mucosal concentrations of angiogenic factors and gastric disease progression in H. pylori-infected Koreans, particularly in the IL-8-251 AA genotype (14). However, several studies conducted in Western countries did not find any significant association between IL-8-251 polymorphism and risk of gastric cancer (29, 30). In this study, significant difference in angiogenic factor levels was caused by H. pylori eradication, not by the IL-8 polymorphism. These results suggest that the IL-8-251 polymorphism had relatively little influence on angiogenic factor levels compared with the effect of H. pylori.
This study had some limitations. Data were retrospectively reviewed and patients were not equally randomized either to receive H. pylori eradication therapy or not after endoscopic resection of gastric neoplasm. Furthermore, H. pylori-eradication failure group was too small to analyze the effect of the IL-8-251 polymorphism. Most of the studies suggesting the association between IL-8-251 polymorphism and gastric carcinogenesis included a lot more cases (3, 4, 13). More well-designed studies based on larger subjects are needed to clarify our results.
In conclusion, it is suggested that elevated IL-8 and MMP-9 concentrations in H. pylori-infected gastric mucosa are altered significantly after successful eradication of H. pylori and these conditions continue for 18 months follow-up period. However, IL-8-251 polymorphism does not affect H. pylori eradication rate and the sequential changes of related angiogenic factors after H. pylori eradication in Koreans.
Figures and Tables
 | Fig. 1Comparison of the H. pylori-eradicated, H. pylori-infected and H. pylori-eradication failure group in relation to histological grade of the antrum, according to the IL-8-251 A/T polymorphism. Scores of (A) neutrophil, (B) monocyte infiltration, (C) atrophy and (D) intestinal metaplasia. Results are shown as the mean ± S.E.M. *P < 0.05 when compared with baseline histology. |
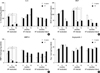 | Fig. 2Comparison of the H. pylori-eradicated, H. pylori-infected and H. pylori-eradication failure group in relation to concentration of IL-8, VEGF, MMP-9, and Ang-1, according to the IL-8-251 A/T polymorphism. Concentrations of (A) IL-8, (B) VEGF, (C) MMP-9 and (D) Ang-1. Results are shown as the mean ± S.E.M. *P < 0.05 when compared with baseline concentrations. |
References
1. Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001. 345:784–789.
2. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007. 133:659–672.
3. Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005. 14:2487–2493.
4. Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, Koike T, Sekine H, Ohara S, Shimosegawa T. The polymorphism interleukin 8-251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005. 54:330–335.
5. Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005. 26:631–636.
6. Machado JC, Figueiredo C, Canedo P, Pharoah P, Carvalho R, Nabais S, Castro Alves C, Campos ML, Van Doorn LJ, Caldas C, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003. 125:364–371.
7. El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003. 124:1193–1201.
8. Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002. 94:1680–1687.
9. Zhang QB, Dawodu JB, Husain A, Etolhi G, Gemmell CG, Russell RI. Association of antral mucosal levels of interleukin 8 and reactive oxygen radicals in patients infected with Helicobacter pylori. Clin Sci (Lond). 1997. 92:69–73.
10. Craig PM, Territo MC, Karnes WE, Walsh JH. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992. 33:1020–1023.
11. Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, Yasui W, Aihara M, Imagawa K, Haruma K, Chayama K. Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun. 2003. 311:809–814.
12. Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, Yasui W, Ohmoto Y, Kajiyama G, Fidler IJ, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998. 152:93–100.
13. Ye BD, Kim SG, Park JH, Kim JS, Jung HC, Song IS. The Interleukin-8-251 A allele is associated with increased risk of noncardia gastric adenocarcinoma in Helicobacter pylori-infected Koreans. J Clin Gastroenterol. 2009. 43:233–239.
14. Song JH, Kim SG, Jung SA, Lee MK, Jung HC, Song IS. The interleukin-8-251 AA genotype is associated with angiogenesis in gastric carcinogenesis in Helicobacter pylori-infected Koreans. Cytokine. 2010. 51:158–165.
15. Cox JM, Clayton CL, Tomita T, Wallace DM, Robinson PA, Crabtree JE. cDNA array analysis of cag pathogenicity island-associated Helicobacter pylori epithelial cell response genes. Infect Immun. 2001. 69:6970–6980.
16. Arinir U, Klein W, Rohde G, Stemmler S, Epplen JT, Schultze-Werninghaus G. Polymorphisms in the interleukin-8 gene in patients with chronic obstructive pulmonary disease. Electrophoresis. 2005. 26:2888–2891.
17. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996. 20:1161–1181.
18. Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005. 37:990–993.
19. Arima N, Adachi K, Katsube T, Amano K, Ishihara S, Watanabe M, Kinoshita Y. Predictive factors for metachronous recurrence of early gastric cancer after endoscopic treatment. J Clin Gastroenterol. 1999. 29:44–47.
20. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004. 291:187–194.
21. Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004. 53:1244–1249.
22. Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002. 16:1449–1456.
23. Yeo M, Kim DK, Han SU, Lee JE, Kim YB, Cho YK, Kim JH, Cho SW, Hahm KB. Novel action of gastric proton pump inhibitor on suppression of Helicobacter pylori induced angiogenesis. Gut. 2006. 55:26–33.
24. Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005. 54:1536–1540.
25. Kamada T, Haruma K, Hata J, Kusunoki H, Sasaki A, Ito M, Tanaka S, Yoshihara M. The long-term effect of Helicobacter pylori eradication therapy on symptoms in dyspeptic patients with fundic atrophic gastritis. Aliment Pharmacol Ther. 2003. 18:245–252.
26. Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, Leung WK, Ng EK, Lau JY, Lee YT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000. 119:7–14.
27. Bergin PJ, Anders E, Sicheng W, Erik J, Jennie A, Hans L, Pierre M, Qiang PH, Marianne QJ. Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter. 2004. 9:201–210.
28. Kim JS, Kim JM, Jung HC, Song IS. Helicobacter pylori down-regulates the receptors of vascular endothelial growth factor and angiopoietin in vascular endothelial cells: implications in the impairment of gastric ulcer healing. Dig Dis Sci. 2004. 49:778–786.
29. Savage SA, Hou L, Lissowska J, Chow WH, Zatonski W, Chanock SJ, Teager M. Interleukin-8 polymorphisms are not associated with gastric cancer risk in a Polish population. Cancer Epidemiol Biomarkers Prev. 2006. 15:589–591.
30. Kamangar F, Abnet CC, Hutchinson AA, Newschaffer CJ, Helzlsouer K, Shugart YY, Pietinen P, Dawsey SM, Albanes D, Virtamo J, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control. 2006. 17:117–125.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download