Abstract
Ceftriaxone is widely used in patients for the treatment of serious gram-negative infections. Ceftriaxone can induce some potential side effects, including neurotoxicity, however, nonconvulsive status epilepticus has rarely been reported. We report a case of acute reversible neurotoxicity associated with ceftriaxone. A 65-yr-old woman with chronic kidney disease developed altered consciousness during ceftriaxone treatment for urinary tract infection. The electroencephalogram demonstrated continuous bursts of generalized, high-voltage, 1 to 2 Hz sharp wave activity. Neurologic symptoms disappeared following withdrawal of ceftriaxone. The possibility of ceftriaxone-induced neurotoxicity should be considered in patients developing neurological impairment during ceftriaxone use, and the discontinuation of the drug could lead to complete neurological improvement.
Ceftriaxone is a third-generation cephalosporin, commonly used in the treatment of serious gram-negative infections due to its broad antimicrobial spectrum, long half-life, and easy penetration into the cerebrospinal fluid (1, 2). Contrary to other cephalosporins, a dose adjustment is not required for ceftriaxone even in the presence of renal insufficiency, thus it is prescribed conveniently for patients with chronic kidney disease (CKD) (3). Neurologic adverse effects of ceftriaxone are infrequent, however, encephalopathy, myoclonus, and seizures may occur as exemplified in other cephalosporins (4-6). Here, we describe a case of ceftriaxone-induced nonconvulsive status epilepticus (NCSE) with characteristic electroencephalogram (EEG) findings and discuss with literature review.
A 65-yr-old woman with CKD was admitted to our hospital because of abdominal pain and poor oral intake on November 18, 2010. She had been diagnosed with idiopathic membranous glomerulonephropathy on renal biopsy six months prior to admission. Her abdominal discomfort began two weeks earlier and was located predominantly in the right lower quadrant, and was associated with lower back discomfort. She described a constant pressure unrelated to food intake that was associated with intermittent nausea and vomiting. Her symptoms were not relieved by the administration of antacid, acetaminophen, or defecation. Laboratory data at patient admission were as follows: serum creatinine 3.2 mg/dL (1.7 mg/dL, 8 weeks before admission), albumin 1.8 g/dL (2.7 g/dL, 8 weeks before admission), and random urine protein-to-creatinine ratio 10.4 g/g (3.3 g/g, 8 weeks before admission), which demonstrated the deterioration of renal function and increase in urinary protein excretion. Gastroscopy exhibited superficial gastritis and the colonoscopy revealed no observable mucosal lesions. To identify other potential causes of abdominal pain and exacerbation of renal insufficiency, such as renal vein thrombosis, computed tomography (CT) scan of abdomen and pelvis was recommended, however she refused further work up being afraid of hemodialysis following CT scan. On day 15 of the hospitalization, she developed a chill, and her temperature rose to 38.5℃. Because she complained of vague urinary symptoms and urinalysis demonstrated pyuria and bacteriuria, ceftriaxone (2 g/day) was administered intravenously for empirical treatment of urinary tract infection. Her temperature returned to normal after three days. On day 20 of the patient's hospitalization, her mental status deteriorated suddenly into stupor and generalized myoclonic jerks were appeared. There was no history of seizures, and no other relevant neurological features observed upon neurological examination. The patient exhibited no fever and the C-reactive protein was 3.2 mg/dL (normal range < 0.3). The serum creatinine level rose to 6.7 mg/dL and the daily urine amount decreased to 300 mL, therefore an emergent hemodialysis was initiated for progressive uremia. Her consciousness did not improve despite 24-hr intensive hemodialysis. A Brain CT demonstrated no abnormalities to explain these neurologic findings. An EEG recording on the next day showed generalized slowing with superimposed almost continuous or periodic bursts of sharp waves or sharp and slow wave activity (Fig. 1A). Under the impression of ceftriaxone-induced NCSE, ceftriaxone was discontinued on day 22 of the hospitalization, and she returned to a completely alert, cooperative, and oriented state within two days of cessation of the drug. The EEG on day 24 showed slowing of the background without epileptiform discharges (Fig. 1B). In addition, the abdomen/pelvis CT revealed extensive thrombosis at the inferior vena cava, both renal veins, right ovarian vein, and both external iliac veins (Fig. 2), thus anticoagulation was started. The patient was discharged on day 58 without any neurologic signs or symptoms.
In this report, we described a case of NCSE following the administration of ceftriaxone in a patient with progressive renal impairment. The temporal relationship between the start of ceftriaxone therapy and the manifestation of NCSE as well as the withdrawal of ceftriaxone and the disappearance of the symptom strongly indicated that ceftriaxone was a causative agent. Moreover, hemodialysis did not improve her mental status, and we could not find any other factors that could explain the neurologic symptom observed.
Neurotoxicity has been reported with both third-generation and fourth-generation cephalosporins (7-9). Epileptogenic activity of β-lactam antibiotics was first shown in 1945, when seizures were reported in experimental animals following intraventricular injection of penicillin (10), particularly in the settings of renal failure and excessive dosage (11). The exact mechanism is not fully understood, but it has been proposed to be mediated by competitive antagonism of brain γ-aminobutyric acid (GABA), which is the principal inhibitory neurotransmitter in the brain (12). Inhibition of GABA action could lead to a low neuronal threshold and subsequent excitation. Other authors (13) have proposed that cephalosporins might induce the release of cytokines including tumor necrosis factor-α, which could cause direct cerebral toxicity.
Cephalosporin-induced neurotoxicity may manifest in a variety of clinical presentations, such as encephalopathy or mental status changes, myoclonus, asterixis, and seizures (4-8). The latency of neurotoxicity, the period between the start of cephalosporin treatment and the resulting neurologic manifestations, varied between one and ten days. All reported neurologic symptoms typically resolved within two to seven days after discontinuation of the drug. The ceftriaxone-associated neurologic syndrome observed in our patients was similar to studies previously described in the literature (6-8). Predisposing factors for cephalosporins-induced neurotoxicity include excessive dosage, renal insufficiency, pre-existing central nervous system (CNS) abnormalities, and increased cerebral penetration of the drug (12, 14, 15). Different EEG patterns have been described in association with cephalosporin neurotoxicity. EEG findings include diffuse slow-wave delta activity, semi-periodic triphasic sharp wave activity, or frank periodic discharges (7). These EEG abnormalities serve as evidence supporting the impact of cephalosporins on the CNS. Given that the majority of patients using cephaloporins have co-morbidity associated with mental alteration, EEG could be of benefit in the diagnosis and management of antibiotics-associated neurotoxicity.
The prevention of cephalosporin-induced neurotoxicity in high risk patients, as discussed above, is of the utmost importance. The careful monitoring of medication dosages and serum levels may be helpful in the minimization of this risk (7). As seen in this case report, many symptoms resolved completely only with discontinuation of the drug. Anticonvulsants such as phenytoin or valproate were administered in two previously reported cases, and their symptoms improved five days after stopping ceftriaxone (4). Besides, we did not use any anticonvulsants because of the rapid (< 2 days) clinical improvement after discontinuation of ceftriaxone. Data are still lacking as to whether patients with cephalosporin-induced NCSE require antiepileptic drug therapy or simple cessation of drugs results in improvement. However, some authors recommend that anticonvulsant therapy including lorazepam and/or phenytoin might be considered in NCSE until the patient's mental status returns to normal and periodic discharges or NCSE shown on EEG resolve (7). Since neurotoxicity related to cephalosporins is reversible, it is unlikely that patients would need long-term anticonvulsant therapy.
Ceftriaxone has a distinctive pharmacokinetic property unlike other cephalsporins (3). While most of cephalosporins are highly dialyzable (70% of a given dose over a 3-hr hemodialysis session), ceftriaxone is not dialyzed during hemodialysis (3, 7). Moreover, the pharmacokinetics of ceftriaxone in patients with renal insufficiency differ markedly from those of other cephalosporins. Compared to values in healthy subjects, the half-life of other cephalosporins is prolonged 6- to 8-fold in patients with end-stage renal disease. However, the half-life of ceftriaxone is not greatly affected by a progressive decrease in the renal function (3). Accordingly, dose adjustment of ceftriaxone is not required for CKD patients. These pharmacokinetic characteristics might be related to the characteristics of ceftriaxone neurotoxicity. Only seven cases of ceftriaxone-induced neurotoxicity have been reported to date (Table 1). All the previous cases had renal impairment and 4 cases of those cases were on hemodialysis. There were 4 cases of choreoathetosis, 2 cases of NCSE, and one case of encephalopathy. Notably, there was no case of which the observed neurologic symptoms and signs were improved by dialysis. These findings are compatible with the pharmacokinetic property of ceftriaxone that is not removable by dialysis. Moreover, given that no dose adjustment is required for ceftriaxone in the patient with renal impairment, overdose did not seem to play a role in ceftriaxone-associated neurotoxicity. In the previous reports, the elimination half-life of ceftriaxone was markedly prolonged in some dialysis patients, it might be due to an impaired biliary excretion process that is the alternative pathway of elimination for ceftriaxone in CKD patients (7,16). These might contribute to the development of infrequent ceftriaxone neurotoxicity in patients with renal insufficiency.
Ceftriaxone can cause NCSE in patients with impaired renal function. This neurotoxicity should be considered carefully in patients with reduced renal function or prior CNS abnormalities. In addition, EEG should be performed when a patient receiving ceftriaxone develops neurological symptoms. Early recognition is of the utmost importance because the discontinuation of ceftriaxone reverts the symptoms completely.
Figures and Tables
 | Fig. 1Electroencephalogram (EEG) findings during nonconvulsive status epilepticus and recovery state. (A) EEG on day 22, when the patient presented an altered mental state. An EEG showed generalized slowing of the background with superimposed almost continuous bursts of generalized, moderate to high amplitude that had an almost periodic pattern (arrows). (B) EEG on day 24, when the patient's neurologic symptoms resolved completely. Epileptiform discharges had disappeared. |
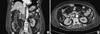 | Fig. 2Abdomen/Pevis CT showing the extensive thrombosis (arrowheads). (A) Thrombosis in the inferior vena cava. (B) Thrombosis in the renal vein. |
Table 1
Summary of reported cases of ceftriaxone-induced neurotoxicity

*Latency is the period between the start of ceftriaxone treatment and the onset of neurologic features; †Days to improvement are the period between the cessation of ceftriaxone and improvement of neurologic findings. RI, renal impairment; CVA, cerebro vascular accident; UTI, urinary tract infection; NCSE, non-convulsive status epilepticus; NA, not available; AED, anti-epileptic drug.
References
1. Rockowitz J, Tunkel AR. Bacterial meningitis. Practical guidelines for management. Drugs. 1995. 50:838–853.
2. Weisfelt M, de Gans J, van de Beek D. Bacterial meningitis: a review of effective pharmacotherapy. Expert Opin Pharmacother. 2007. 8:1493–1504.
3. Patel IH, Sugihara JG, Weinfeld RE, Wong EG, Siemsen AW, Berman SJ. Ceftriaxone pharmacokinetics in patients with various degrees of renal impairment. Antimicrob Agents Chemother. 1984. 25:438–442.
4. Martinez-Rodriguez JE, Barriga FJ, Santamaria J, Iranzo A, Pareja JA, Revilla M, dela Rosa CR. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am J Med. 2001. 111:115–119.
5. Sato Y, Morita H, Wakasugi H, Iijima S, Kawashima E, Wakayama Y, Yoshimura A. Reversible chreoathetosis after the administration of ceftriaxone sodium in patients with end-stage renal disease. Am J Med Sci. 2010. 340:382–384.
6. Roncon-Albuquerque R Jr, Pires I, Martins R, Real R, Sousa G, von Hafe P. Ceftriaxone-induced acute reversible encephalopathy in a patient treated for a urinary tract infection. Neth J Med. 2009. 67:72–75.
7. Grill MF, Maganti R. Cephalosporin-induced neurotoxicity: clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann Pharmacother. 2008. 42:1843–1850.
8. Lam S, Gomolin IH. Cefepime neurotoxicity: case report, pharmacokinetic considerations, and literature review. Pharmacotherapy. 2006. 26:1169–1174.
9. Kim SY, Lee IS, Park SL, Lee J. Cefepime neurotoxicity in patients with renal insufficiency. Ann Rehabil Med. 2012. 36:159–162.
10. Johnson H, Walker A. Intraventricular penicillin: a note of warning. JAMA. 1945. 127:217–219.
11. De Sarro A, Ammendola D, Zappala M, Grasso S, De Sarro GB. Relationship between structure and convulsant properties of some beta-lactam antibiotics following intracerebroventricular microinjection in rats. Antimicrob Agents Chemother. 1995. 39:232–237.
12. Wallace KL. Antibiotics-induced convulsions. Crit Care Clin. 1997. 13:741–762.
13. Alkharfy KM, Kellum JA, Frye RF, Matzke GR. Effect of ceftazidime on systemic cytokine concentrations in rats. Antimicrob Agents Chemother. 2000. 44:3217–3219.
14. Schliamser SE, Cars O, Norrby R. Neurotoxicity of beta-lactam antibiotics: predisposing factors and pathogenesis. J Antimicrob Chemother. 1991. 27:405–425.
15. Calandra G, Lydick E, Carrigan J, Weiss L, Guess H. Factors predisposing to seizures in seriously ill infected patients receiving antibiotics: experience with imipenem/cilastatin. Am J Med. 1988. 84:911–918.
16. Cohen D, Appel GB, Scully B, Neu HC. Pharmacokinetics of ceftriaxone in patients with renal failure and in those undergoing hemodialysis. Antimicrob Agents Chemother. 1983. 24:529–532.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download