Abstract
We report on an angioedema patient with a genetic defect in complement 1 inhibitor, manifesting migraine-like episodes of headache, effective prophylaxis with Danazol, and triptan for a treatment of acute clinical episode. The patient was 44-yr-old Korean man with abdominal pain and headache, who was brought into the Emergency Department of Seoul National University Hospital, Seoul. He suffered from frequent attacks of migraine-like headache (3-7 per month), pulsating in nature associated with nausea. Severities were aggravated by activity and his headache had shown recent progression with abdominal pain. No remarkable findings were observed on radiologic examination, brain magnetic resonance images and intracranial and extracranial magnetic resonance angiography. Danazol 200 mg every other day was subsequently used. Following administration of Danazol, symptoms showed improvement and the patient was discharged. While taking Danazol, the migraine-like episodes appeared to be prevented for about 2 yr. At the eighth month, he suffered a moderate degree of migraine-like headache; however, administration of naratriptan 2.5 mg resolved his problem. A case of genetic defect of C1-INH deficiency presented with headache episodes, and was controlled by Danazol and triptan. It suggests that pathogenic mechanism of headache in hereditary angioedema may be mediated by the neurogenic inflammatory-like physiology of migraine.
Angioedema is clinically characterized by episodes of marked edema involving the skin, gastrointestinal, and other organs. There are various forms of acquired and hereditary angioedema (HAE). Classic HAE is associated with a deficiency of the Complement 1 inhibitor (C1-INH), C1 esterase. Deficiencies of this protein allow unchecked activation of the classic complement pathway (1). C1-INH also inhibits components of the fibrinolytic, clotting, and kinin pathways. Activation of the kinin system increases blood bradykinin, an inflammatory mediator responsible for capillary leakage. Bradykinin excites primary sensory neurons and provokes the release of neuropeptides such as substance P, neurokinin A, and calcitonin gene-related peptide (2).
In a previous report of headache and HAE, headache were reported in 18 of 209 patients. The severe headache lasted for 4 hr to 4 days, accompanied by other signs; feeling of pressure in the head or eyes, visual disturbances, giddiness, disorders of balance, ataxia, impaired orientation, vomiting, or decrease in physical and mental powers. Headache may be the troublesome symptom of HAE. Thus, all patients reported that analgesics were not effective. Five patients received C1-INH concentrate, which was effective in all treated episodes. Although they presented to be similar to migraine episodes, however, some clinical features typical for migraine were lacking. The prompt response to C1-INH concentrate has provided a further argument that these headache episodes are symptoms of HAE (3).
The first drugs used in treatment of HAE patients were androgens and their attenuated derivatives. High doses of these drugs can cause a significant increase in C1 inhibitor plasma levels (4). Before development of effective therapy, the mortality rate was 20%-30%. Although preventable and treatable, the complications of this disease do not respond well to the usual therapies for angioedema; therefore, establishment of the correct diagnosis is critical.
Several theories regarding the migraine mechanism have been proposed. Vasodilation theory of migraine, originally proposed in 1938, focused on vasodilatation as the predominant causative factor in development of migraine pain. However, cerebral vasodilatation is not temporally correlated with migraine pain using transcranial Doppler, PET, or fMRI; therefore, this theory is not uniformly supported (5-7). According to the neurogenic inflammation theory of migraine, release of inflammatory neuropeptides, like substance P, neurokinin A and CGRP from the trigeminal sensory afferent onto the dura can act on vascular tissue to cause the components of neurogenic inflammation; vasodilation, plasma protein extravasation in the surrounding area, endothelial cell changes, platelet aggregation and subsequent release of serotonin and other mediators, white cell adhesion and subsequent inflammation (8). Vasoconstrictive acute therapeutic agents like ergotamines and triptan also inhibit release of inflammatory neuropeptides (9).
Therefore, it can be postulated that C1-INH deficiency inactivates kinin systems that increase bradykinin. This can provoke the release of inflammatory neuropeptides which mediate neurogenic inflammation of migraine headache.
We report on a patient with a genetic defect in Complement 1 inhibitor, manifesting migraine-like episodes of headache, effective prophylaxis with Danazol, and triptan for a treatment of acute clinical episode.
A 44-yr-old man was brought to the Emergency Department complaining of abdominal pain and headache on October 8, 2009. The patient reported abdominal pain, anorexia, and a severe right temporal pulsating headache for three days. He had been diagnosed as hereditary angioedema (HAE) with deficiency of C1 esterase inhibitor (C1INH). There was family history of HAE in sister, uncle and cousin. Episodic attacks of headache developed 4 yr ago. It was unilateral, either right or left, or of a bilateral pulsating nature. Headache was aggravated by activity and associated with nausea. Conjunctival injection or rhinorrhea was not associated. He denied any visual or other sensory prodromal symptoms before the onset of headache. There had been moderate severity of headache episodes three to seven times per month. Occasionally, he suffered from severe attacks of headache once or twice a year. No remarkable findings were observed on radiologic examination including computed tomography in the abdomen. Brain magnetic resonance image and intracranial and extracranial magnetic resonance angiography did not show any significant abnormalities (Fig. 1). The level of complement C3 was 218 mg/dL and C4 was 7 mg/dL when he had visited emergency room. Danazol 200 mg every other day was medicated. While taking Danazol, the migraine-like attack was not happened for 23 months. At the 8th month, he suffered a moderate nature of migraine-like headache, when naratriptan 2.5 mg was administered. His headache resolved within 2 hr and no further attach thereafter. The case report was approved by the institutional review board in Seoul National University Hospital. Informed consent was exempted by the board.
We report on a patient with a genetic defect in Complement 1 inhibitor, associated with migraine-like headache. Danazol, a synthetic 17 alpha-alkylated androgen derivative has been used for the treatment of HAE (10). It has been also effective for the recurrence of headache episode.
In this case, Danazol administration could prevent symptoms or signs of HAE including severe headache. The headache was also prevented for up to 23 months. The mechanism of Danazol in HAE has been suggested that high doses can cause a significant increase in C1 inhibitor plasma levels (4). It then inhibit the kinin system, which reduces bradykinin, and thus resulting in decreased inflammatory peptide (2). These signaling cascades also overlap with that of neuroinflammatiory roles in the pathophysiology of migraine.
Triptans are agonists of serotonin (5-HT)1B and 5-HT1D receptors and have been used for treatment of migraines that do not respond to NSAIDs (11-13). Triptan also inhibit the release of inflammatory neuropeptides (9). In this patient, Naratriptan (triptan drug) was effective when headache episode appeared. However, response to the naratriptan was not enough to confirm the diagnosis of migraine, or efficacy of other drugs. Neither it excludes other possibilities of multiple mechanisms in migraine pathogenesis. However, present finding with response to naratriptan can suggest that headache associated with HAE can be interpreted as migraine-like headache, possibly involving pathophysiology in neurogenic inflammation. Reported efficacy C1-INH concentrate also supported the neuroinflammatory mechanism of headache in patients with HAE, which may account for the efficacy of Danazol in this case. However, this is only one case-based observation, further evidences are warranted to be accumulated.
It is unknown whether Danazol can be used for the prophylaxis of migraine. Reports on 55 Vietnamese immigrants with headache showed that there was decreased level of C1 esterase inhibitor. Although all of them did not have angioedema, Danazol treatment improved headache, and these patients may represent a form of androgen-responsive headache, which is associated with low levels of C1INH (14). Incidence of headache or migraine in HAE is lower than that of migraine in general population. It can be assumed that Danazol treatment for HAE may likely reduce the attack of headache in patient comorbid with migraine headache. Gender-difference with more female preponderance can also be considered to be associated with this hormonal effect, which remains to be explored (15).
Taken together, a case of genetic defect of C1-INH deficiency presented with migraine-like headach, and was controlled by Danazol and triptan, suggesting the neurogenic inflammatory mechanism in migraine physiology.
Figures and Tables
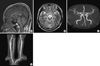
Fig. 1
Brain MRI and MR angiography of this patient. (A) T1-weighted saggital magnetic resonance image shows no significant brain parenchymal abnormality. There is no evidence of subdural fluid collection or other mass lesion. (B) T2-weighted axial scan shows symmetric apprearence of cerebral hemisphere without remarkable findings in suprasellar cistern. (C) Intracranial vessels including, internal carotid arteries, middle cerebral arteries and its bifurcation, anterior cerebral arteries, vertebrobasilar arteries do not show any stenosis or occlusion. (D) Neck vessels, subclavian artery, common carotid arteries with bifurcation and origin of bilateral vertebral arteries are within normal limit.
References
1. Pappalardo E, Zingale LC, Terlizzi A, Zanichelli A, Folcioni A, Cicardi M. Mechanisms of C1-inhibitor deficiency. Immunobiology. 2002. 205:542–551.
2. Goodman LS, Gilman A, Brunton LL. Goodman & Gilman's manual of pharmacology and therapeutics. 2008. New York: McGraw-Hill Medical.
3. Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006. 119:267–274.
4. Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976. 295:1444–1448.
5. Limmroth V, May A, Auerbach P, Wosnitza G, Eppe T, Diener HC. Changes in cerebral blood flow velocity after treatment with sumatriptan or placebo and implications for the pathophysiology of migraine. J Neurol Sci. 1996. 138:60–65.
6. Woods RP, Iacoboni M, Mazziotta JC. Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med. 1994. 331:1689–1692.
7. Welch KM, Cao Y, Aurora S, Wiggins G, Vikingstad EM. MRI of the occipital cortex, red nucleus, and substantia nigra during visual aura of migraine. Neurology. 1998. 51:1465–1469.
8. Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci. 1987. 7:4129–4136.
9. Buzzi MG, Carter WB, Shimizu T, Heath H 3rd, Moskowitz MA. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991. 30:1193–1200.
10. Agostoni A, Cicardi M, Martignoni GC, Bergamaschini L, Marasini B. Danazol and stanozolol in long-term prophylactic treatment of hereditary angioedema. J Allergy Clin Immunol. 1980. 65:75–79.
11. Johnston MM, Rapoport AM. Triptans for the management of migraine. Drugs. 2010. 70:1505–1518.
12. Tepper SJ, Rapoport AM, Sheftell FD. Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol. 2002. 59:1084–1088.
13. Moon HS, Chu MK, Park JW, Oh K, Chung JM, Cho YJ, Kim EG, Do JK, Jung HG, Kwon SU. Frovatriptan is effective and well tolerated in Korean migraineurs: a double-blind, randomized, placebo-controlled trial. J Clin Neurol. 2010. 6:27–32.
14. Luong KV, Nguyen LT. Headache and complement C1-esterase inhibitor deficiency in Vietnamese immigrants living in southern California. Allergy Asthma Proc. 1999. 20:127–133.
15. Kang EH, Park JE, Chung CS, Yu BH. Effect of biofeedback-assisted autogenic training on headache activity and mood states in Korean female migraine patients. J Korean Med Sci. 2009. 24:936–940.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download