Abstract
Indeterminate cytology results increase the number of repetitive procedure and unnecessary surgery. This study was designed to find useful and simple predictive tools to differentiate malignant thyroid nodules from indeterminate nodules. We retrospectively enrolled 164 patients who had undergone thyroid surgery as a result of indeterminate cytology in the National Cancer Center. We reviewed patients' age at diagnosis, sex, preoperative biochemical markers such as thyroglobulin (Tg), anti-Tg antibody, free T4 and TSH level, and sonographical and pathological findings, which were subjected to statistical analysis. We found several clinical and sonographical predictive factors that showed significant differences. Young age, male, preoperative high Tg level, and hypoechoic nodule on sonography all increased cancer probability significantly in multivariate analysis. With a cut-off value of 187.5 ng/mL Tg, sensitivity and specificity were 54.8% and 90.1%, respectively (AUC 0.748, P < 0.001). In the case of nodule size > 1.7 cm, elevated serum Tg predicts the risk of malignancy; especially Tg > 70 ng/mL (odds ratio 3.245, 95% confidence interval 1.115-9.450, P = 0.038). Preoperative Tg levels had very high specificity in predicting thyroid cancer in case of suspicious follicular neoplasm. Therefore, Tg levels may be a useful marker for differentiating thyroid cancer from benign thyroid nodules in the cytological diagnosis of indeterminate nodules.
Thyroid nodules are very common (1), and most do not impact life expectancy of the affected person (2). However, along with increased detection rates of non-palpable thyroid nodules, the incidence rate of thyroid cancer has been raised (3, 4) and the absolute number of indeterminate thyroid nodule cytology results has also markedly increased. Although fine-needle aspiration cytology (FNAC) is a primary method for detecting malignant nodules, 10%-25% of thyroid nodules are categorized as indeterminate nodules (5). Classifications for indeterminate nodules are defined as "atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS)" and "follicular neoplasm (FN) or suspicious for a follicular neoplasm (SFN)" under the Bethesda classification systems (6), and the risk of malignancy of the FN/SFN category nodules is approximately 15%-30%. These renewed classifications also cannot resolve the difficulties clinicians face in differentiating malignant from benign nodules.
Numerous studies were performed in order to differentiate benign and malignant thyroid nodules using clinical factors other than cytology. Unfortunately, there are currently no useful differentiating markers except for some well-known risk factors such as positive family history, tumor size, and age (7-10).
Therefore, we tried to elucidate the role of preoperative serum thyroglobulin (Tg) as a novel marker that could distinguish between benign and malignant thyroid nodules, especially in the rigorously selected FN/SFN category.
We reviewed FNAC results from 789 patients who underwent thyroid surgery at the National Cancer Center from May 2002 to July 2009 and selected 164 cases compatible with indeterminate cytology results according to the new Bethesda system category (Fig. 1) (6). One pathologist re-examined the patient cytology and pathology slides under the criteria as followed; 1) FN/SFN: follicular cells are arranged predominantly in microfollicular or trabecular arrangements whether nuclear atypia may or may not be present, 2) AUS/FLUS : neither convincingly benign nor sufficiently atypical to place into a different category (11). In our analysis we selected 164 cases showing cytology results of FN/SFN category and also reviewed patients' clinical, biochemical, sonographical and pathological findings. We excluded the cases whose cytological results have been reported as another category (benign or malignancy) after repeated aspirations.
The serum TSH levels were measured by an immunoradiometric assay (IRMA) using a commercial kit (Immunotech, Marseille, France). Tg and anti-Tg antibody (TgAb) levels were measured using IRMA and a commercial radioimmunoassay kit, respectively (Brahms Aktiengesellschaft, Hennigsdorf, Germany). The reference ranges for TSH, Tg and TgAb were 0.17-4.05 mU/L, 0-40 ng/mL and 0-100 U/mL, respectively.
We obtained patients' sex, age at diagnosis and TSH, Tg, and TgAb levels from medical records. Reviewing ultrasonography, cytology slide, and pathology specimens, we also obtained tumor size, characteristics of tumor such as margin, component, echogenicity, hypoechoic rim, vascularity, calcification and presence of Hashimoto's thyroiditis and final pathological diagnosis. Hashimoto's thyroiditis was defined as diffuse thyroid disease measured by ultrasonography and/or positive TgAb or microsomal antibody (12, 13).
We grouped patients into the benign or cancer groups (papillary thyroid carcinoma [PTC] or follicular thyroid carcinoma [FTC]) according to the final diagnosis. Continuous variables are expressed as means ± standard deviation (SD), whereas categorical variables are presented as absolute values and percentages. Differences between continuous variables were analyzed by unpaired Student's t-test or ANOVA, and differences between categorical variables were analyzed using the chisquare test. Multivariate and logistic regression analysis was employed to identify independent predictors of malignancy. An ROC curve was plotted to determine the Tg cut-off levels for differentiating cancerous from benign nodules. A P value of < 0.05 was considered statistically significant, and all analyses were performed using Stata statistical software, version 9 (Stata Corporation, College Station, TX, USA).
First, we compared basal clinical findings between benign and malignant groups that had shown indeterminate nodule cytology results. The benign group and malignant group comprised 88 (53.7%) and 76 (46.3%) of the total patients, respectively. Malignant groups were composed of 32 conventional PTCs, 9 follicular variant PTC (FVPTC), 31 minimally invasive FTC (MIFTC), and 4 widely invasive FTC (WIFTC). Benign groups included 53 follicular adenoma, 32 nodular hyperplasia, 2 hyperplastic nodules, and 1 Hashimoto's thyroiditis.
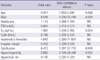
Table 1 shows the results of comparison between groups. Patients' sex, age at diagnosis and TSH levels did not differ between groups. The size of tumors was larger in FTCs and smaller in PTCs than in benign nodules (benign vs MIFTC vs WIFTC, 2.4 ± 1.2 vs 2.9 ± 1.1 vs 4.3 ± 1.3 cm, P = 0.001; benign vs PTC, 2.4 ± 1.2 vs 0.8 ± 0.1 cm, P < 0.001, benign vs FVPTC, 2.4 ± 1.2 vs 1.2 ± 0.7 cm, P = 0.025). Besides tumor size, preoperative Tg levels were higher in the FTC group than in the benign group: benign vs MIFTC vs WIFTC, median (range), 15.4 (1-1,499) vs 188.0 (2.3-7,940) vs 2,078.5 ng/mL (31.7-6,860), P < 0.001.
When we compared sonographical findings between groups, we found that irregular margin, low echogenicity, absence of hypoechoic rim and presence of calcification were detected more commonly in malignant thyroid nodules (Table 2).
We also performed multivariate analysis to find predictive factors of thyroid malignancy. As shown in Table 3, younger age, being male, higher Tg levels, hypoechoic nodules and the presence of calcification were independent and significant risk factors for FTC.
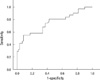
We performed ROC curve analyses to find cut-off levels of age at diagnosis, tumor size, and Tg to differentiate the benign and malignant groups. Age at diagnosis, using a cut-off value of 52.5 yr, had a sensitivity of 48.5% and a specificity of 68.2% for detecting the malignant group with an area under the curve (AUC) of 0.524 (95% confidence interval [CI] 0.404-0.645, P = 0.674), which failed to show significant differences between groups (data not shown). Mean tumor size, using a cut-off of 1.7 cm, had a sensitivity of 85.7% and a specificity of 32.3% with an AUC of 0.663 and P = 0.005 (95% CI 0.562-0.764) (data not shown). Preoperative Tg levels (AUC 0.748, 95% CI 0.634-0.861, P < 0.001) showed a sensitivity of 48.5% and a high specificity of 91.5% with cut-off value of 187.5 ng/mL (Fig. 2).
The risk of malignancy was increased in nodules more than 1.7 cm in size; especially in the case of Tg > 70 ng/mL, the odds ratio [OR] was 3.245 (95% CI 1.115-9.450, P = 0.038), and the sensitivity and specificity was 67.7% and 60.7%, respectively. Without consideration of nodule size, the nodule with preoperative Tg > 100 ng/mL showed increased risk (OR 2.913, 95% CI 1.134-7.483, P = 0.029).
We compared the clinical, biochemical, and ultrasonographical findings between benign follicular adenomas and carcinomas. We found that preoperative Tg cut-off levels of 187.5 ng/mL might predict increased risk of malignancy and discriminate between benign and malignant nodules (AUC 0.748, 95% CI 0.634-0.861, P < 0.001).
Thyroid nodules are very common and have recently begun to be detected more frequently (1, 14). Ultrasonography-guided fine needle aspiration (FNA) is a widely used, cost effective, and accurate diagnostic tool for thyroid cancer. However, over 20% of patients undergoing FNA of a thyroid nodule have indeterminate cytology results and usually require thyroid operation for the purpose of definite diagnosis. Pathologic diagnosis results in benign nodules for most patients, and if the patients are diagnosed with thyroid cancer, they should undergo completion thyroidectomy (15).
The malignancy rate of indeterminate cytology was assumed to be 20%-30% (6, 14, 16), however, Granados-Garcia et al. (17) also reported high rate of malignancy (40%, 30/75). Recently, Rossi et al. (18) also reported 34.9% of malignancy risk in indeterminate cytology even though it had conducted as a prospective study. Similarly, percentage of malignancy was relatively high (46.3%, 46/164) in our study. Considering that we excluded patients who did not undergo surgery, high malignancy rate may be thought to lie in acceptable range.
Lots of studies were performed to classify indeterminate nodules into benign and malignant groups. Young or old age, male gender, nodule size, family history of thyroid cancer (19), and history of benign nodule (20) are known to be associated with a high risk of malignant thyroid nodule. However, until now, there have been no studies showing any definite differentiating risk factor. In addition, risk factors of FTC are still not well understood except low iodine intake (21). Only a few recent studies such as studies of Kim et al. (9) and Sippel et al. (8) suggested tumor size and age as risk factors of Hurthle cell thyroid carcinoma.
We explored rigorously several clinical, biochemical, and sonographical findings; young age and male gender were independent risk factors for predicting high risk of FTC similar to previous findings (Table 3, OR, [95% CI], P value; old vs young age, 0.917 [0.850-0.990], P = 0.026; male vs female, 8.036 [1.230-52.499], P = 0.030) (15, 16). Interestingly, preoperative Tg levels were independent risk factors of FTC (OR 1.987, 95% CI 1.248-3.165, P = 0.004, Table 3). Nodule size, however, was not different between the benign and cancer groups after multivariate analysis (OR 1.116, 95% CI 0.698-1.783, P = 0.683) in our study. These insignificant differences may come from characteristics of retrospective study itself; higher preference to surgery in patients with larger nodules, which was the limitation of our study.
Serum Tg levels can be elevated in most proliferative thyroid diseases and are known to be an insensitive and nonspecific test for thyroid cancer. Routine measurement of serum Tg levels for initial evaluation of thyroid nodules is not recommended (7). Our result was also compatible with previous reports in PTC or FVPTC groups; that is, for most prevalent thyroid cancer cases, preoperative Tg levels did not differ from those of benign nodules (Table 1, benign vs FVPTC vs PTC, 15.4 [1-1499] vs 9.3 [2.4-398] vs 8.8 ng/mL [0.3-181]). Recently, there were two reports which lead opposite conclusions about the efficacy of measurement of preoperative serum Tg; Sands et al. (22) reported that a combination of indeterminate cytology and preoperative Tg ≥ 75 ng/mL increased diagnostic efficacy compared to indeterminate cytology alone and suggested that elevated preoperative Tg levels may be predictive of well-differentiated thyroid cancer (benign vs cancer, Tg levels; 223 vs 53 ng/mL, P = 0.007). However, Suh et al. (23) concluded that Tg has poor accuracy for predicting malignancy in follicular or Hurthle cell neoplasms. These studies were conducted in smaller population than that of our study, and the experimental approach was different from ours.
In our study, preoperative Tg levels were significantly higher in the FTC group, and according to the severity of follicular cancer, Tg levels increased steeply (Table 2, benign vs MIFTC vs WIFTC, Tg levels, [95% CI], P value; 15.4 [1-1,499] vs 188.0 [2.3-7,940] vs 2,078.5 ng/mL [31.7-6,860], P < 0.001). ROC curves also showed good sensitivity and specificity for the cut-off value 187.5 ng/mL (AUC 0.748, 95% CI 0.634-0.861, P < 0.001, sensitivity of 48.5%, and specificity of 91.5%).
Taken together, the above results indicate that preoperative Tg levels may be one of the most useful single predictors for distinguishing benign nodules from FTCs.
In summary, preoperative Tg levels had very high specificity in predicting FTC in the case of indeterminate nodules. Therefore, in practice, preoperative Tg measurement may be a very useful single tool for differentiating FTCs from benign thyroid nodules in the case of indeterminate cytology results.
Figures and Tables
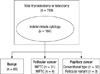 | Fig. 1Patients enrolled in our study. Indeterminate cytology included all of "atypical of undetermined significance or follicular lesion of undetermined significance" and "follicular neoplasm or suspicious for a follicular neoplasm." MIFTC, minimally invasive follicular thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma. |
References
1. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994. 154:1838–1840.
2. Rago T, Chiovato L, Aghini-Lombardi F, Grasso L, Pinchera A, Vitti P. Non-palpable thyroid nodules in a borderline iodine-sufficient area: detection by ultrasonography and follow-up. J Endocrinol Invest. 2001. 24:770–776.
3. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
4. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.
5. Rosen JE, Stone MD. Contemporary diagnostic approach to the thyroid nodule. J Surg Oncol. 2006. 94:649–661.
6. Cibas ES, Ali SZ. The Bethesda System for reporting thyroid cytopathology. Thyroid. 2009. 19:1159–1165.
7. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.
8. Sippel RS, Elaraj DM, Khanafshar E, Zarnegar R, Kebebew E, Duh QY, Clark OH. Tumor size predicts malignant potential in Hurthle cell neoplasms of the thyroid. World J Surg. 2008. 32:702–707.
9. Kim TH, Lim JA, Ahn HY, Lee EK, Min HS, Kim WK, Choi YH, Park YJ, Park DJ, Kim KH, et al. Tumor size and age predict the risk of malignancy in Hurthle cell neoplasm of the thyroid and can therefore guide the extent of initial thyroid surgery. Thyroid. 2010. 20:1229–1234.
10. Ron E, Kleinerman RA, Boice JD Jr, LiVolsi VA, Flannery JT, Fraumeni JF Jr. A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987. 79:1–12.
11. Bongiovanni M, Cibas ES, Faquin WC. The role of thyroid fine needle aspiration cytology and the Bethesda system for reporting thyroid cytopathology. Diagn Histopathol. 2011. 17:95–105.
12. Ciccone MM, De Pergola G, Porcelli MT, Scicchitano P, Caldarola P, Iacoviello M, Pietro G, Giorgino F, Favale S. Increased carotid IMT in overweight and obese women affected by Hashimoto's thyroiditis: an adiposity and autoimmune linkage? BMC Cardiovasc Disord. 2010. 10:22.
13. Schmidt M, Voell M, Rahlff I, Dietlein M, Kobe C, Faust M, Schicha H. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto's thyroiditis) treated with levothyroxine. Thyroid. 2008. 18:755–760.
14. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993. 118:282–289.
15. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of "follicular neoplasm": a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002. 26:41–44.
16. Carpi A, Nicolini A, Gross MD, Fig LM, Shapiro B, Fanti S, Rampin L, Polico C, Rubello D. Controversies in diagnostic approaches to the indeterminate follicular thyroid nodule. Biomed Pharmacother. 2005. 59:517–520.
17. Granados-Garcia M, Cortes-Flores AO, del Carmen Gonzalez-Ramirez I, Cano-Valdez AM, Flores-Hernandez L, Aguilar-Ponce JL. Follicular neoplasms of the thyroid: importance of clinical and cytological correlation. Cir Cir. 2010. 78:473–478.
18. Rossi M, Buratto M, Bruni S, Filieri C, Tagliati F, Trasforini G, Rossi R, Beccati MD, Degli Uberti EC, Zatelli MC. Role of ultrasonographic/clinical profile, cytology, and BRAF V600E mutation evaluation in thyroid nodule screening for malignancy: a prospective study. J Clin Endocrinol Metab. 2012. 97:2354–2361.
19. Hemminki K, Eng C, Chen B. Familial risks for nonmedullary thyroid cancer. J Clin Endocrinol Metab. 2005. 90:5747–5753.
20. Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, McTiernan A, Kolonel L, Mark SD, Mabuchi K, et al. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control. 1999. 10:583–595.
21. Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid. 2002. 12:889–896.
22. Sands N, Karls S, Rivera J, Tamilia M, Hier MP, Black MJ, Gologan O, Payne RJ. Preoperative serum thyroglobulin as an adjunct to fine-needle aspiration in predicting well-differentiated thyroid cancer. J Otolaryngol Head Neck Surg. 2010. 39:669–673.
23. Suh I, Vriens MR, Guerrero MA, Griffin A, Shen WT, Duh QY, Clark OH, Kebebew E. Serum thyroglobulin is a poor diagnostic biomarker of malignancy in follicular and Ḧurthle-cell neoplasms of the thyroid. Am J Surg. 2010. 200:41–46.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download