Abstract
Menkes disease is an infantile-onset X-linked recessive neurodegenerative disorder caused by diverse mutations in a copper-transport gene, ATP7A. Affected patients are characterized by progressive hypotonia, seizures, failure to thrive and death in early childhood. Here, we report a case of Menkes disease presented by intractable seizures and infantile spasms. A 3-month-old male infant had visited our pediatric clinic for lethargy, floppy muscle tone, poor oral intake and partial seizures. His hair was kinky, brown colored and fragile. Partial seizures became more frequent, generalized and intractable to antiseizure medications. An EEG showed frequent posteriorly dominant generalized spikes that were consistent with a generalized seizure. From a genetic analysis, a c.2743C>T (p.Gln915X) mutation was detected and diagnosed as Menkes disease. The mutation is a novel one that has not been previously reported as a cause of Menkes disease.
Menkes disease (OMID 309400), also known as kinky hair disease, is an infantile-onset X-linked recessive neurodegenerative disorder caused by diverse mutations in a copper-transport gene, ATP7A (1, 2). The ATP7A gene plays an important role in controlling copper efflux from cells (3). Affected patients appear healthy at birth and develop normally for 6 to 8 weeks. Subsequently, hypotonia, seizures, failure to thrive and death in early childhood are typical (4, 5). At present, a total of 170 different mutations have been identified worldwide (6).
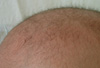
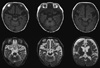
A 3-month-old male infant had visited our pediatric clinic for lethargy, floppy muscle tone, poor oral intake and partial seizures on May 9, 2007. He was born at 38 weeks gestation by vaginal delivery. His birth weight was 3,180 g. He was healthy at birth and a neonatal metabolic screening test was negative. His hair was kinky, brown colored and fragile (Fig. 1). Symptoms of seizure were characterized by left facial twitching and left arm clonic movements. The initial EEG showed one episode of 2 Hz rhythmic spike and wave activity starting from the right central area evolving to the generalized slowings lasting about 100 seconds without clinical seizures, which was consistent with electrical partial seizures (Fig. 2A). There was no significant acidosis and ammonia was not increased. Serum lactate, tandem mass screening, serum amino acid and urine organic acids were all within the normal range. Pyruvate level (0.263 mM/L, reference range: 0.03-0.08 mM/L) was high. Partial seizures became more frequent and intractable to antiepileptic medications. About 1 month later, generalized flexor spasms developed. At this time, the EEG had changed to irregular high amplitude delta slowings on the background activities and frequent spikes from the right or left frontal or occipital areas that were consistent with hypsarrhythmia (Fig. 2B). Vascular tortuosity and diffuse brain atrophy with callosal thinning were detected in an MRI scan (Fig. 3) at 3.5 months. Biochemical markers showed low serum copper (9.0 µg/dL, reference range: 70-130 µg/dL) and ceruloplasmin (5.6 mg/dL, reference range: 16-31.5 mg/dL) levels. From genetic analysis, a c.2743C>T (p.Gln915X) missense mutation (Fig. 4) in exon 13 of the ATP7A gene was detected, and the infant was diagnosed with Menkes disease (MD). The mutation was a novel one that has not been previously reported as a cause of MD. He died at 13 months of life.
We analyzed the ATP7A gene in a Korean patient with classical MD and identified one novel mutation. The ATP7A gene at Xq13.3 contains 23 exons and encodes a copper-transporting P-type ATPase of 1500 amino acids (3). To date, about 170 different mutations affecting ATP7A have been reported (6, 7). Approximately 25% of the ATP7A mutations are gross deletions, ranging in size from a single exon to deletion of the whole gene, except for the first two exons (6). About 120 other different intragenic mutations of ATP7A have been reported: missense (33%), nonsense (16%), splice-site mutations (16%) and deletions/insertions/duplications (33%) (Human Gene Mutation Database [HGMD]; www.hgmd.com) (7).
The biochemical result of low copper concentrations in MD is reduced activity of numerous copper-dependent enzymes such as ceruloplasmin, dopamine beta-hydroxylase, peptidylglycine alpha-amidating monooxygenase, cytochrome C oxidase, ascorbate oxidase, lysyl oxidase, superoxide dismutase and tyrosinase, which leads to connective tissue abnormalities, tortuosity of blood vessels and peculiar hair (1, 8). The phenotypic features of Menkes disease can be divided into at least three categories: classical MD with death in early childhood, mild MD with long survival and occipital horn syndrome (9). The majority of patients suffer from classical MD, but milder forms are observed in 5%-10% of patients. There seems to be poor genotype-phenotype correlation, and the clinical courses of MD patients may differ within a family, despite identical genetic changes (10).
Epilepsy is one of the main features of MD. Based on recent studies, the development of epilepsy can be divided into three phases: 1) an early stage characterized by focal clonic status, usually triggered by fever; 2) an intermediate stage with intractable infantile spasms, in which interictal EEG demonstrated modified hypsarrhythmia, with diffuse irregular slow waves, and spike waves; and 3) a late stage with multifocal seizures, tonic spasms and myoclonus (11, 12).
Treatment in major cases is mainly symptomatic and supportive. However, neonatal diagnosis by plasma neurochemical measurement before symptoms appear and early parental copper-histidine supplement may modify the disease progression substantially (13-15). Prenatal diagnosis can be performed by biochemical analysis or DNA assay using chorionic villi samples or amniocytes in the first trimester of an at-risk pregnancy (16, 17).
In summary, we report a case of Menkes disease presented by intractable seizures and infantile spasms because of a novel missense mutation (c.2743C>T) in the ATP7A gene.
Figures and Tables
References
1. Kodama H, Murata Y. Molecular genetics and pathophysiology of menkes disease. Pediatr Int. 1999. 41:430–435.
2. Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993. 3:20–25.
3. Voskoboinik I, Camakaris J. Menkes copper-translocating P-type ATPase (ATP7A): biochemical and cell biology properties, and role in Menkes disease. J Bioenerg Biomembr. 2002. 34:363–371.
4. Bacopoulou F, Henderson L, Philip SG. Menkes disease mimicking non-accidental injury. Arch Dis Child. 2006. 91:919.
5. Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993. 3:7–13.
6. Tümer Z, Birk Møller L, Horn N. Screening of 383 unrelated patients affected with Menkes disease and finding of 57 gross deletions in ATP7A. Hum Mutat. 2003. 22:457–464.
7. Møller LB, Bukrinsky JT, Mølgaard A, Paulsen M, Lund C, Tümer Z, Larsen S, Horn N. Identification and analysis of 21 novel disease-causing amino acid substitutions in the conserved part of ATP7A. Hum Mutat. 2005. 26:84–93.
8. Kim OH, Suh JH. Intracranial and extracranial MR angiography in Menkes disease. Pediatr Radiol. 1997. 27:782–784.
9. Møller LB, Tümer Z, Lund C, Petersen C, Cole T, Hanusch R, Seidel J, Jensen LR, Horn N. Similar splice-site mutations of the ATP7A gene lead to different phenotypes: classical Menkes disease or occipital horn syndrome. Am J Hum Genet. 2000. 66:1211–1220.
10. Møller LB, Mogensen M, Horn N. Molecular diagnosis of Menkes disease: genotype-phenotype correlation. Biochimie. 2009. 91:1273–1277.
11. Bahi-Buisson N, Kaminska A, Nabbout R, Barnerias C, Desguerre I, De Lonlay P, Mayer M, Plouin P, Dulac O, Chiron C. Epilepsy in Menkes disease: analysis of clinical stages. Epilepsia. 2006. 47:380–386.
12. White SR, Reese K, Sato S, Kaler SG. Spectrum of EEG findings in Menkes disease. Electroencephalogr Clin Neurophysiol. 1993. 87:57–61.
13. George DH, Casey RE. Menkes disease after copper histidine replacement therapy: case report. Pediatr Dev Pathol. 2001. 4:281–288.
14. Choi JH, Yoo HW. Long term treatment of copper-histidine in a Menkes disease patient. J Korean Soc Inherit Metab Dis. 2004. 4:34–39.
15. Kaler SG, Holmes CS, Goldstein DS, Tang J, Godwin SC, Donsante A, Liew CJ, Sato S, Patronas N. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008. 358:605–614.
16. Poulsen L, Horn N, Heilstrup H, Lund C, Tümer Z, Møller LB. X-linked recessive Menkes disease: identification of partial gene deletions in affected males. Clin Genet. 2002. 62:449–457.
17. Choi JH, Kim GH, Yoo HW. Identification of novel mutations of the ATP7A gene and prenatal diagnosis of Menkes disease by mutation analysis. J Genet Med. 2007. 4:38–44.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download