Abstract
Endothelin (ET)-1 and its receptors (ETA and ETB receptor) are present in the central nervous system. ET exerts biological effects on gliogenesis and glial cell functions. In order to define a possible mechanism of ETA receptor signaling, the distribution of the ETA receptor in developing oligodendrocytes and the effects of ET-1 on the myelination of oligodendrocytes were examined. ETA receptor immunoreactivity was confined to the perivascular elements of the blood vessels during early postnatal development. However later in development, ETA receptor immunoreactivity was no longer observed in the vessels but became localized to the myelinating oligodendrocytes of the primitive corpus callosum of the white matter, apart from the vessels. ET-1 induced myelin basic protein (MBP) in primary oligodendrocyte precursor cell culture though the ETA receptor and was blocked by an ETA receptor antagonist. In addition, ET-1 evoked the release of Ca2+ which is a central regulator of oligodendrocyte differentiation. Our results provide a link between ET-1 and its ETA receptor and myelination during oligodendrocyte differentiation.
The endothelins (ETs) are 21 amino acid peptides that were originally cloned from bovine aortic endothelial cells (1) and that are expressed as three biologically active peptides: ET-1, ET-2, and ET-3. ETs are expressed by a variety of cell types including endothelial cells, macrophages, astrocytes, and neurons (2). ET isoforms act through the G protein-coupled receptors, the endothelin A (ETA) receptor and the endothelin B (ETB) receptor. Different ET peptides modulate the development of distinct neural cell types including Schwann cells (3), astrocytes (4), and neural crest cells (5) as well as physiologic renal growth and development (6). ET receptor expression is regulated during neural cell maturation. Each ET isoform can act through both of the receptors but the receptors have different affinities for each of the 3 isoforms. The ETA receptor has a greater affinity for ET-1 and the ETB receptor binds to all 3 peptides equally (7). This distinction between ligand binding and receptor activation is thought to contribute to the diversity of their physiological responses (8, 9).
The findings that ETs exert biological effects on gliogenesis and glial cell function raise the important question of whether this peptide might affect oligodendrocyte development. Oligodendrocytes are thought to originate in a restricted ventricular zone of the ventral neural tube and then migrate to their final destinations and mature there (10). Recently, Gallo and colleagues (11) reported that ET-1 is a soluble astrocyte-derived signal that can regulate the migration and developmental differentiation of oligodendrocyte precursor cells (OPCs). However, they did not show the specific stages of regulation on OPC differentiation and related signaling pathways. In addition, the role of ET-1 on models of autoimmune demyelination like experimental autoimmune encephalomyelitis (EAE) was studied by blocking the action of ET-1 with an ETA receptor antagonist, BQ-123. Intrathecal administration of BQ-123 significantly ameliorates EAE progression at its peak stage (12).
In this study, in order to define a possible role for ET-1 in oligodendrocyte development, we morphologically examined ETA receptor expression during each stage of oligodendrocyte development and the functional role of ETA receptor in oligodendrocyte myelination.
Brains were removed from ICR mice (Samtako, Osan, Korea) on postnatal day (P) 1 and 1, 2, 4, and 8 weeks of age (i.e., P1d, P1w, P2w, P4w and P8w, n = 5 in each group), and then fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) at pH 7.4 for 2 hr. All animal experiments were approved by the Institutional Animal Care and Use Committee in Chungnam National University (2009-3-17). The tissue was cryoprotected by infiltration with a 10%-30% sucrose solution, embedded in an Optimal Cutting Temperature compound, and then rapidly frozen in 2-methyl butane precooled to its freezing point with liquid nitrogen. Tissue specimens were cut into 10 µm sagittal or horizontal sections on a cryostat and then mounted. Immunohistochemical staining of the tissue sections was done using the avidin-biotin peroxidase complex (ABC) method described previously (13). Sections were treated with blocking buffer (1% fetal bovine serum and 0.3% Triton X-100 in PBS) for 30 min after endogenous peroxidase blocking with 1% H2O2 in PBS and then rinsed thoroughly with PBS. Sections were incubated with the primary antibody for 24 hr at 4℃. The antibodies and their dilutions were ETA receptor (anti-rabbit, Sigma, St. Louis, MO, USA, E3651, 1:100), CD31 (anti-rat, BD Biosciences, Frankin Lakes, NJ, USA, 550274, 1:500), myelin basic protein (MBP, anti-rat, Millipore, Billerica, MA, USA, MAB386, 1:100), and adenomatous polyposis coli protein (APC, anti-mouse, Merck, Darmstadt, Germany, OP80, 1:500). Tissues were exposed to the biotinylated anti-mouse IgG and the streptavidin peroxidase complex (Vector labs, Burlingame, CA, USA) for 1 hr at room temperature after rinsing with PBS. Immunostains were visualized with diaminobenzidine (DAB, Sigma) for 3-5 min and then mounted using Polymount. Double immunofluorescent experiments were done using Cy™2-conjugated anti-rabbit IgG (1:400) and Cy™3-conjugated anti-mouse IgG (1:600, Amersham, Buckinghamshire, UK). All immunoreactions were examined under a fluorescent microscope (IX71, Olympus, Tokyo, Japan).
We prepared primary OPC cultures by mechanical dissociation according to the method by Chen et al. (14). Briefly, a P1 Sprague-Dawley rat pup (Samtako, Osan, Korea) was decapitated in an ice-chilled dish and the brain was removed. Mixed cultures were shaken overnight to detach OPCs from the astrocyte monolayer. Oligodendrocyte progenitors were plated on poly-D-lysine-coated culture dishes and grown in basal defined media (BDM) consisting of DMEM20S (DMEM, 4 mM L-glutamine, 1 mM sodium pyruvate, 20% FBS), 0.1% bovine serum albumin, 50 µg/mL human transferrin, 10 nM hydrocortisone, 10 nM biotin, 5 µg/mL insulin and 30 nM selenium. After stabilization of the oligodendrocyte progenitor cells for 3 days, 20 ng/mL each of PDGFAA and bFGF (Peprotech, Rocky Hill, CT, USA) were added to the media to differentiate the OPCs which were used after 3 days in vitro (DIV). The cultured cells were characterized immunocytochemically with cell-type-specific antibodies. More than 95% of the cells were positive for the monoclonal antibody A2B5 (clone 105, IgM, R&D systems, Minneapolis, MN, USA) and for the PDGFA receptor (CD140a, Millipore), a marker for OPCs in culture, while less than 5% of the cells were GalC-positive OLGs, GFAP-positive astrocytes or complement type-3-positive microglia (15, 16).
Total RNA was extracted from cultured OPCs using TRizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The amount of RNA and its purity was determined by measuring the optical density at 260 nm and the optical density ratio at 260 and 280 nm (ratio ~ 2), respectively. RNA integrity was checked by electrophoresis on agarose gel. Approximately 10 µg of total RNA from each sample was used to generate cDNA in a 40 µL reaction using 200 U of the SuperScript II (Invitrogen, Carlsbad, CA, USA) reverse transcriptase and oligo (dT). Subsequently, 2 µL of cDNA was subjected to PCR amplification in order to measure the expression of MBP gene. To amplify rat MBP, we used the following forward primer: 5'-CTGCCCTCGGCTTCTTAAT-3' (456-474) and reverse primer 5'-GTTTTAGCCAGTCAGGGTGC-3' (576-595). The nucleotide position reported in brackets corresponded to the published rat MBP cDNA sequence (GenBank accession number, M25889). Genes were amplified as follows: denaturation at 94℃ for 1 min, annealing at 60℃ for 1 min, extension at 72℃ for 1 min, and this was repeated for 28 cycles. PCR products were resolved by 1.2% agarose gel electrophoresis and visualized under UV light.
At P1d, P1w, P2w, P4w and P8w, the ICR mice (Samtako) were anesthetized. The entire brain was removed quickly from each mouse and homogenized in lysis buffer (50 mM TrisCl, 150 mM NaCl, 0.02% sodium azide, 100 µL/mL PMSF, 1 µg/mL aprotinin, 1% Triton X-100). Supernatant protein concentrations were determined with Micro BCA protein assay kits. Bovine serum albumin was used as a standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing 20 µg of protein were boiled in 2 × sample buffer (0.8 M TrisCl, 10% glycerol, 20% β-mercaptoethanol, 10% SDS, and 0.02% bromophenol blue) for 5 min and loaded onto a polyacrylamide gel. Proteins were transferred onto nitrocellulose membranes after electrophoresis at 250 mA for 1 hr. Membranes were incubated in 5% skim milk in PBST (0.3% Triton X-100 in PBS) for 1 hr to block non-specific binding and then probed with a primary antibody: ETA receptor (1:500), MBP (1:500), and β-actin (1:5,000, Sigma). Membranes were washed 3 times for 10 min in PBST and incubated for 1 hr with a peroxidase-labeled secondary antibody (Vector Labs, Burlingame, CA, USA) diluted 1:2,000 in PBST. Immunolabeled proteins were detected by chemiluminescence after 3 additional washes using a Supersignal ECL kit (Pierce Chemical, Rockford, IL, USA) and Biomax Light-1 films. For blotting of OPCs, cells were treated with ET-1 and/or BQ123 and then harvested and cell extracts were obtained as above. Primary antibodies for ETA-receptor (1:500) and MBP (1:500) were used and detected as above.
OPCs grown on coverslips were loaded with the Ca2+ indicator Fura-2/AM according to the manufacturer's instructions (Molecular Probes, Carlsbad, CA, USA). Images were obtained by confocal microscopy (Zeiss LSM510 Meta; Carl Zeiss, Jena, Germany). For kinetic measurements, Ca2+-free HBSS buffer solution containing 10 mM EGTA was used to eliminate the extracellular Ca2+, and images were obtained every 5 sec for 15 min at 37℃ using a Cool-SNAP microscope (HQ, Photometrics, Tucson, AZ, USA).
During early development (P1d), ETA receptor immunoreactivity has been associated with blood vessels (Fig. 1A). However later in development, ETA receptor immunoreactivity was no longer observed in the vessels but became localized to the round oligodendrocyte-like cells of the corpus callosum and the white matter, apart from the vessels (Fig. 1B-D). MBP immunoreactivity was weakly found in the primitive corpus callosum region and then intensified in the corpus callosum during later development (Fig. 1E-H). ETA receptor immunoreactivity was observed on the exterior surface of blood vessels and appeared as small, oval cell bodies with processes that extended for some distance parallel to the vessel axes (Fig. 1I). To examine the localization of the ETA receptor in the perivascular elements of the vessels, double labeling with the ETA receptor and CD31, a marker of endothelial cells, was done. The ETA receptor was associated with blood vessels, but its perivascular elements were found to not co-localize with CD31, suggesting that the ETA receptor was expressed in the perivascular cells during early development (Fig. 2A-C). Since ETA receptor immunoreactivity was found in the primitive corpus callosum, double labeling with MBP, a marker of mature oligodendrocytes, was done. The ETA receptor co-localized with MBP positive cells at P1w (Fig. 2D-F) and the amount of ETA receptor-positive labeling in oligodendrocytes was higher at later stages of development. In addition, labeling with the other oligodendrocyte marker APC was also confirmed (Fig. 2G-I). Western blot was done to examine changes in the ETA receptor and MBP levels. Intense ETA receptor bands were detected at P1d and the intensity of these bands increased in ages approaching adulthood (P8w). On the other hand, MBP levels were found at P2w and they increased thereafter. Immunoblots for these ETA receptor proteins in the developing brain generally confirm the immunochemical findings (Fig. 3).
We first examined whether treatment of OPCs with an ETA receptor agonist, ET-1, would lead to increased oligodendrocyte myelination. OPCs treated with ET-1 exhibited an aggregation of cells and induction of MBP which is indicative of myelination (Fig. 4). To further examine the effect of ET-1 and ETA receptors on the myelination of oligodendrocytes, we measured MBP gene expression after 48 hr of treatment with ET-1. The RT-PCR analysis of MBP mRNA by a MBP-specific primer (17) amplified a band of 140 bp. As expected, the application of ET-1 dramatically increased MBP expression (Fig. 5A). The ET-1 response was totally abolished when cells were pretreated with BQ123, an ETA receptor antagonist. A relative estimate of PCR products was carried out by evaluating the ratio of MBP and the corresponding GAPDH bands for each template. Next, we used a pharmacological approach to show that ETA receptors are activated by ET-1 during oligodendrocyte myelination. MBP protein was elevated with ET-1 and down-regulated with BQ123 (Fig. 5B). The data suggest that ET-1 increases the myelination of oligodendrocytes by activating the endogenously expressed main ETA receptor subtype.
It has been shown that Ca2+ signaling is essential in the development and functioning of oligodendrocytes. For example, Ca2+ uptake is required for the initiation of myelination (18). Therefore, we examined the effect of ET-1 on calcium release kinetics with a Cool-SNAP microscope. A 4-fold increase in fluorescence intensity was detected within 2 min after ET-1 stimulation in normal bath solution (HBSS with normal [Ca2+]), which went down immediately and out of phase at 3 min (Fig. 6). This data suggests that ET-1 stimulation induces the myelination of oligodendrocytes through Ca2+ release.
In the present study, we investigated the role of the ETA receptor in the myelination of OPCs. Our data showed that the ETA receptor is expressed in the capillary wall during early postnatal development, but during later development, the ETA receptor is expressed in the myelinating oliogodendrocytes. We also demonstrated that ET-1 induces the myelination of oligodendrocytes by the ETA receptor and subsequent Ca2+ release is a central regulator of ETA receptor-mediated oligodendrocyte myelination.
Despite the widespread distribution of ET system components throughout the brain (19), their function in oligodendrocytelineage cells was unexplored. Gadea et al. (11) reported that ET receptors were expressed from the immature oligodendrocyte progenitor stage to the differentiated phenotype stage. The constitutive expression of the ETB receptor early in development and the upregulation of the ETA receptor under culture conditions which induced OPC differentiation suggest that the ETB receptor is expressed as multipotential progenitors and neural stem cells whereas the ETA receptor is upregulated in committed progenitors and their differentiated progenies. However, this study did not show the role that the ETA receptor has on specific stages of oligodendrocyte development in vivo. In this study, we clearly showed that the ETA receptor was expressed in myelinating oligodendrocytes of the white matter.
Gallo and colleagues (11) also reported that ET-1 affected OPC differentiation by delaying the transition from the O4 to the more differentiated O1 stage. In addition, they showed that ET-1 reduced MBP and CNP levels at all times in culture and promoted migration and decreased the differentiation rate of OPCs. However, these findings appear to contrast with our results. We confirmed several times that ET-1 induced MBP levels in OPC cultures. This discrepancy between the previous study and our results could be due to the different method of culturing the OPCs, e.g., the presence or absence of putrescine, progesterone, T3 hormone, etc., because thyroid treatment initiates differentiation of oligodendrocytes into myelinating oligodendrocytes (20). In addition, OPCs arise from an overlapping domain with the somatic motor neurons and migrate to and mature in their final microenvironments (10, 21). Migratory OPCs are unipolar or bipolar in shape and become highly branched in their target region (21, 22). Therefore, ETA receptor expression in newly emerging myelinating oligodendrocytes in the primitive corpus callosum suggests that ETA receptors might play an important role in the differentiation of premyelinating to myelinating oligodendrocytes.
In the normal spinal cord, ETA receptor immunoreactivity is found in vascular smooth muscle cells and in a subpopulation of primary afferent nerve fibers (23). Moreover in dorsal root ganglion and peripheral nerves, ETA receptor immunoreactivity is present in a subset of small-sized peptidergic and nonpeptidergic sensory neurons and their axons and to a lesser extent in a subset of medium-sized sensory neurons (24). Since these reports about the expression of ETA receptors were different with our results, we tested the other antibodies (Alomone Labs, Jerusalem, Israel and Research Diagnostics Inc., Flanders, NJ) that were used in the above papers. As a result, we found a similar expression of ETA receptors in the developing oligodendrocytes as in our original study.
Development of oligodendrocytes involves migration of OPCs to their final destinations within the CNS, followed by local proliferation, and finally OPCs either differentiate into myelin-forming oligodendrocytes or die by apoptosis (10, 25). These processes are regulated by extracellular and axon-dependent factors, many of which mediate their actions through multiple Ca2+-dependent intracellular signaling pathways (26). Downstream targets of Ca2+ include extracellular signal-regulated kinases and cyclic AMP response element binding protein (CREB), which regulate oligodendrocyte cell proliferation, survival, growth, and differentiation (27). Extracellular Ca2+ influx through voltage-independent Ca2+ channels plays a critical role in ET-1-induced cell proliferation and vascular contraction (28). Biological actions of ET-1 are mediated by ETA and ETB which belong to a family of G-protein-coupled receptors (7). It has been shown that the Gq subfamily induces Ca2+ entry not only by indirect store-operated mechanisms but also by direct stimulation of cation channels (29). These results, taken together, suggest that ET-1 might induce the differentiation of oligodendrocytes through ETA receptor-mediated Ca2+ signaling.
In this study, we showed that the effects of ETA receptor in the myelination of oligodendrocytes act through Ca signaling. We suggest that ETA receptor-mediated signaling by ET-1 might play a role in oligodendrocyte myelination. Our findings on ET-1 and oligodendrocyte myelination are important for remyelination strategies in demyelinating diseases of the central nervous system. Although OPCs are present in demyelinated regions, it is still undetermined why their differentiation and ability to remyelinate are inhibited in diseases such as multiple sclerosis. We previously reported that activated CREB is expressed in a myelin-associated protein in chicks (13). To elucidate the Ca signaling pathway via protein kinase C and CREB, further research is required.
Figures and Tables
Fig. 1
ETA receptor and MBP immunoreactivity with cresyl violet counterstain in the developing mouse brain. Ages examined were P1d (A, E, I), P1w (B, F, J), P2w (C, G, K), and P4w (D, H, L). During early development (P1d), ETA receptor immunoreactivity was associated with blood vessels (A). However at a later point in time, ETA receptor immunoreactivity disappears from the vessels (arrowheads in B), and is instead localized within round oligodendrocyte-like cells in the corpus callosum (CC) (C, D). MBP immunoreactivity appears at P1w in the primitive corpus callosum (arrows in F) and is strongly expressed in the corpus callosum later (G, H). Higher magnification of the rectangular area in A shows the perivascular expression of the ETA receptor (white arrowhead in I) at P1d, but ETA receptor immunoreactivity appears in primitive corpus callosum (arrows in J) and increases with development in oligodendrocyte-like cells of the white matter (K, L). Scale bar = 100 µm in A-H, 20 µm in I-L.
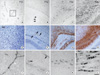
Fig. 2
Fluorescent double labeling of the ETA receptor and CD31, MBP and APC in the developing mouse brain. Double labeling for the ETA receptor (A, D, G) and CD31 (B), MBP (E) and APC (H) was examined at P1d (A-C), P1w (D-F) and P4w (G-I). The ETA receptor is associated with blood vessels and the perivascular elements is co-localized with CD31, a marker of endothelial cells (arrow in C) during early development. At a later point in time, the ETA receptor is co-localized with MBP expression (arrowheads in F). In addition, ETA receptor immunoreactivity is found with another mature oligodendrocyte marker, APC (G-I). Scale bar = 20 µm.

Fig. 3
Western blots for ETA receptor and MBP expression in mice brains during development. This figure shows a representative immunoblot from 3 independent experiments. ETA receptor expression is found at P1d and increased with further development. MBP expression is weakly found at P2w and increased at P4w and P8w.

Fig. 4
Fluorescent double labeling for the ETA receptor and MBP in primary OPCs after ET-1 treatment. The ETA receptor was expressed in a few cells of OPC cultures (A-C). Myelination was assessed by immunostaining with an antibody against MBP, a marker of mature oligodendrocytes. OPCs were treated with ET-1 (100 nM). ETA receptor/MBP positive cells increase at 24 and 48 hr after treatment with ET-1 (D-I). Scale bar = 20 µm.
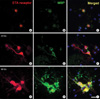
Fig. 5
Western blot for MBP in primary OPCs after ET-1 treatment alone, or with an ETA receptor blocker. OPCs were treated with ET-1 (100 nM) alone and then preincubated with BQ123 (2 µM) for 2 hr before exposure to ET-1. Transcripts (A) and protein (B) of MBP increase after 48 hr of treatment with ET-1 and are blocked by the specific ETA receptor antagonist, BQ123. Data are expressed as optical densities and represent means ± SEM of three independent experiments. *P < 0.05.

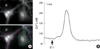
Fig. 6
Effect of ET-1 on intracellular Ca2+ release in primary OPCs. (A) OPCs were loaded with Fura-2/AM and stimulated with ET-1 (100 nM), then imaged by confocal microscopy at 1 min intervals. (B) Graphs show changes in mean fluorescence intensity from 15 cells per microscopic field over time. The figure is representative of three independent experiments. Fura-2/AM was excited with the 340 nm/380 nm, and its fluorescence emission was recorded at 510 nm. The actual number of cells in each case was approximately 100 single cells.
AUTHOR SUMMARY
The Role of Endothelin Receptor A during Myelination of Developing Oligodendrocytes
Kyung Jin Jung, Dong Woon Kim, Ha Na Lee, Young Sook Lee, Sung Joong Lee, Jeong-Hwan Che, Young Ho Lee, and Byeong-Cheol Kang
Factors capable of promoting oligodendrocyte precursor cells (OPCs) differentiation and myelination in vivo is an important therapeutic strategy for a variety of pathologies in which demyelination is a component, including multiple sclerosis and spinal cord injury. Although OPCs are present in demyelinated regions, it is still undetermined why their differentiation and ability to remyelinate are inhibited in diseases such as multiple sclerosis. In this study, we showed that the effects of endothelin A (ETA) receptor in the myelination of oligodendrocytes act through Ca signaling. We suggest that ETA receptor-mediated signaling by ET-1 might play a role in oligodendrocyte myelination.
References
1. Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988. 6:S188–S191.
2. Simonson MS. Endothelins: multifunctional renal peptides. Physiol Rev. 1993. 73:375–411.
3. Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006. 13:627–638.
4. Li JJ, Wu LH, Cao Q, Yuan Y, Yang L, Guo ZY, Kaur C, Sivakumar V, Ling EA, Wu CY. Endothelins-1/3 and endothelin-A/B receptors expressing glial cells with special reference to activated microglia in experimentally induced cerebral ischemia in the adult rats. Neuroscience. 2010. 167:665–677.
5. Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008. 21:598–610.
6. Yoo KH, Yim HE, Jang GY, Bae IS, Choi BM, Hong YS, Lee JW. Endothelin A receptor blockade influences apoptosis and cellular proliferation in the developing rat kidney. J Korean Med Sci. 2009. 24:138–145.
7. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990. 348:730–732.
8. Avedanian L, Riopel J, Bkaily G, Nader M, D'Orleans-Juste P, Jacques D. ETA receptors are present in human aortic vascular endothelial cells and modulate intracellular calcium. Can J Physiol Pharmacol. 2010. 88:817–829.
9. Grantcharova E, Reusch HP, Beyermann M, Rosenthal W, Oksche A. Endothelin A and endothelin B receptors differ in their ability to stimulate ERK1/2 activation. Exp Biol Med (Maywood). 2006. 231:757–760.
10. Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010. 119:37–53.
11. Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J Neurosci. 2009. 29:10047–10062.
12. Shin T, Kang B, Tanuma N, Matsumoto Y, Wie M, Ahn M, Kang J. Intrathecal administration of endothelin-1 receptor antagonist ameliorates autoimmune encephalomyelitis in Lewis rats. Neuroreport. 2001. 12:1465–1468.
13. Kim DW, Chang JH, Park SW, Jeon GS, Seo JH, Cho SS. Activated cyclic AMP-response element binding protein (CREB) is expressed in a myelin-associated protein in chick. Neurochem Res. 2005. 30:1133–1137.
14. Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007. 2:1044–1051.
15. Cohen RI, Almazan G. Rat oligodendrocytes express muscarinic receptors coupled to phosphoinositide hydrolysis and adenylyl cyclase. Eur J Neurosci. 1994. 6:1213–1224.
16. Radhakrishna M, Almazan G. Protein kinases mediate basic fibroblast growth factor's stimulation of proliferation and c-fos induction in oligodendrocyte progenitors. Brain Res Mol Brain Res. 1994. 24:118–128.
17. Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996. 8:1106–1116.
18. Paez PM, Fulton D, Colwell CS, Campagnoni AT. Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J Neurosci Res. 2009. 87:3259–3266.
19. Nambi P, Pullen M, Feuerstein G. Identification of endothelin receptors in various regions of rat brain. Neuropeptides. 1990. 16:195–199.
20. Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci USA. 2002. 99:3258–3263.
21. Ono K, Bansal R, Payne J, Rutishauser U, Miller RH. Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development. 1995. 121:1743–1754.
22. Kim DW, Park SW, Jeon GS, Seo JH, Golden JA, Cho SS. The multiple dorsoventral origins and migratory pathway of tectal oligodendrocytes in the developing chick. Brain Res. 2006. 1076:16–24.
23. Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003. 180:1–13.
24. Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001. 21:999–1006.
25. Butt AM, Pugh M, Hubbard P, James G. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye (Lond). 2004. 18:1110–1121.
26. Butt AM. Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia. 2006. 54:666–675.
27. Soliven B. Calcium signalling in cells of oligodendroglial lineage. Microsc Res Tech. 2001. 52:672–679.
28. Kawanabe Y, Nauli SM. Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function. Cell Signal. 2005. 17:911–916.
29. Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001. 50:646–657.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download