Abstract
Obesity and the metabolic syndrome are closely related and have become increasingly prevalent in Korea. The cardiovascular disease (CVD) risk factors comprising the metabolic syndrome have previously been associated with increased hypothalamic-pituitary-adrenal axis (HPAA) activity, but the associations have not been extensively examined in non-Caucasian populations. The aim of the present study was to investigate the relationships between cortisol, adiposity and the metabolic syndrome in a Korean population. A total of 1,881 adults participated in the study between January 2001 and February 2008. Sociodemographic data were assessed by questionnaires. Body composition, clinic blood pressures as well as metabolic variables including glucose, insulin, and lipid profile were assessed and analyzed in relation to cortisol levels. Mean age of the participants was 58.7 ± 10.8 yr. Higher levels of cortisol was associated with elevated blood pressure, fasting glucose and total cholesterol in men, and between cortisol and systolic blood pressure, fasting glucose and total cholesterol in women. There was an increased risk for the metabolic syndrome associated with higher cortisol levels in both men (P < 0.001) and women (P = 0.040) adjusting for age and body mass index. Higher cortisol levels are associated with several CVD risk factors and the metabolic syndrome, independent of overall of adiposity level, in Korean men and women.
Glucocorticoids, mediated by activation of the hypothalamic-pituitary-adrenal (HPA) axis, have an impact on metabolic responses, insulin-resistance and lipolysis (1). Glucocorticoids directly impair insulin sensitivity in adipocytes (2) and promote free fatty acid release from mature adipocytes through hormone sensitive lipase (HSL)-mediated lipolysis (3). These events are mediated by Glucocorticoid Receptors (GR). The density of these receptors is higher in intraabdominal visceral than other fat depots, and the activity of cortisol will be accentuated in this particular adipose tissue (4). It is now apparent that tissue-specific changes in cortisol metabolism is primarily increased intracellular glucocorticoid reactivation, catalysed by the enzyme 11-hydroxysteroid dehydrogenase type (HSD)-1 in adipose tissue (5).
Abdominal obesity promotes insulin resistance and is considered a main part of the metabolic syndrome (6, 7). Although cortisol has been implicated as a pathophysiological contributor to idiopathic obesity, circulating cortisol has not consistently been shown to be elevated in obese as compared to normal weight individuals (8). A recent study using both cross-sectional and longitudinal analysis to determine the associations between body composition and serum cortisol concentrations in men showed that weight change drives the changes in cortisol concentrations, rather than vice versa (9).
In addition, studies have shown that cortisol may add to the effect of adiposity on metabolic risk. Ward et al. (10) reported that higher cortisol concentrations increased the risk for cardiovascular disease (CVD) risk factors in a South Asian cohort study, above what could be explained by the effect of adiposity alone. In that study, associations between cortisol concentration and CVD risk factors were also stronger than what has previously been shown in Caucasian populations, despite similar cortisol levels. Asian populations have been shown to have a different fat distribution pattern than Western populations and seem to be more prone to central obesity and metabolic dysregulation even at lower body mass index (BMI) levels (11, 12). However, increased prevalence of overweight and obesity does not fully explain the higher prevalence of the metabolic syndrome in Asian populations (12-14), and the role of glucocorticoids has not been investigated in Korean individuals. We hypothesized that cortisol would be positively and independently associated with increased CVD risk, assessed as components of the metabolic syndrome.
We enrolled 2,219 Korean individuals > 20 yr old who had undertaken medical evaluation at Ajou University Hospital between January 2001 and February 2008. Participants were excluded if they were taking steroid medication or did not provide information about smoking status, alcohol consumption, or physical activity (n = 338) leaving a sample of 1,881 participants (56.1% female).
Sociodemographic characteristics, smoking status, alcohol habits, and activity level were assessed by questionnaire. Socioeconomic status (SES) meant financial affordability; and exercise was defined by moderate or severe vs none or sedentary. Height and weight were measured using bioelectrical impedance analysis (Inbody 3.0, Biospace, Seoul, Korea) following overnight fast. BMI was calculated as weight divided by height squared (kg/m2) (15). Waist circumference was measured between the lower rib and iliac crest by a trained nurse. Blood pressure was measured by a semi-automated blood pressure monitor (TM-2650A, PMS Instruments, Tokyo, Japan) after a rest of at least 15 min.
Venous blood was drawn following an 8-hr overnight fast and 24-hr abstinence from vigorous activity. Fasting glucose, total cholesterol and triglyceride level were assessed with an enzymatic colorimetric method (TBA-200FR, Toshiba, Tokyo, Japan); Serum insulin was determined with an immunoradiometric assay kit (Dnabot, Tokyo, Japan) and cortisol with a RIA method (Diagnostic Products Cooperation, Los Angeles, CA, USA). We calculated the insulin resistance index using the homeostasis model assessment (HOMA) of insulin resistance (16).
We followed the NCEP-ATP III Asian guideline components to define metabolic syndrome, comprised of central obesity (waist circumference ≥ 90 cm for men and ≥ 85 cm for women), blood pressure ≥ 130/80 mmHg, triglyceride ≥ 150 mg/dL, fasting glucose ≥ 110 mg/dL, and high-density lipoprotein cholesterol (men < 40 mg/dL and women < 50 mg/dL). In line with the guidelines, subjects who met three or more of the criteria mentioned above were defined as having the metabolic syndrome (17, 18).
Chi-square test and Student's t-test were used to calculate the difference in mean values of general characteristics and laboratory tests between men and women. Regression analysis was conducted to assess the association between cortisol and CVD risk factors while controlling for age and BMI. Further regression analysis was assessed to evaluate the relation of cortisol concentration to the separate CVD risk variables while simultaneously controlling for confounders such as SES, smoking status, alcohol intake and exercise. Finally, logistic regression analysis was used to evaluate the association of cortisol levels to the metabolic syndrome, controlling for age and BMI. A P < 0.05 was used as cutoff for significance. SPSS in version 12.0 was used for all statistical analysis.
A total of 1,881 individuals were assessed, 826 men (43.9%) and 1,055 women (56.1%) (Table 1). The mean age of the subjects was 58.7 ± 10.8 yr and the mean BMI for all subjects was 24.3 ± 3.3 kg/m2. The mean cortisol level for all subjects was 12.7 ± 4.8 µg/dL. Mean levels (± SD) of study variables are presented by gender in Table 1. There were gender differences on several demographic and clinical variables, including age, waist circumference (WC), BMI, cortisol levels, HDL cholesterol, total cholesterol, and systolic blood pressure). In addition, men had higher alcohol consumption and were more often smokers in comparison to women. Almost 32% of the women and 21% of the men met criteria for the metabolic syndrome.
Regression analysis of cortisol to CVD risk factors while controlling for BMI and age are presented in Table 2. Results showed that there were significant associations between cortisol and SBP, DBP, fasting glucose and total cholesterol in men, and between cortisol and SBP, fasting glucose and total cholesterol in women. Multiple regression analysis showed that after additional adjustment for age, BMI, SES, smoking, alcohol consumption and daily activity, higher cortisol levels remained associated with higher fasting glucose concentrations in both men (P < 0.001) and women (P < 0.002). Cortisol was also significantly associated to SBP in both women and men (P = 0.03 and 0.05 respectively). The association of cortisol with DBP was significant in men (P = 0.04), but not in women (P = 0.43). Cortisol tended to be associated with higher total cholesterol in both men and women (P = 0.06). The association of cortisol to triglycerides, HDL and WC was not significant in either men or women after the additional adjustments.
To test whether BMI moderated the effect of cortisol on CVD risk factors and metabolic syndrome, the interaction term BMI × cortisol was calculated and added to the model. However, these analyses did not show any significant moderating effect of BMI on any of the outcomes (P > 0.10).
Insulin and HOMA measures were only available in a subset of the study participants (n = 633). The mean insulin level was 7.3 ± 4.7 µIU/mL for men (n = 262) and 7.0 ± 4.2 µIU/mL for women (n = 371) respectively. In this group, higher levels of cortisol to be associated with reduced insulin secretion (Table 3). The associations of cortisol to insulin and HOMA were not significant (P = 0.18 and P = 0.87 respectively), adjusting for age and BMI.
There was an increased risk for the metabolic syndrome associated with higher cortisol levels in both men (P = 0.000) and women (P = 0.040) while adjusting for age and BMI (Table 4). This effect remained significant in the men after further adjustments for SES, smoking, alcohol drinking, daily activity (P < 0.001), while it became non-significant in the women (P = 0.13).
Results from the present study suggest that higher levels of fasting cortisol are associated with several CVD risk factors in Korean men and women, independent of overall adiposity level as measured by BMI. Our study also showed that the risk for the metabolic syndrome was greater in individuals with high cortisol levels after adjusting for age and BMI. This finding is consistent with previous research showing that individuals with metabolic syndrome have increased cortisol secretion (19, 20) and cortisol metabolite levels (21). Further adjustment for SES status, smoking, alcohol drinking and daily activity reduced this effect in women, but not in the men.
We found a negative association of cortisol to BMI and WC in the women, and no association between cortisol and BMI or WC in the men. These findings were consistent with previous studies showing that cortisol levels were not elevated in obese as compared to normal weight individuals (8, 9), and also in agreement with the study suggesting a diminished stimulability of the HPA axis in obese individuals (22). Cortisol is released from the subcutaneous adipose tissue by 11beta-HSD1 in humans, and increased enzyme expression in obesity is likely to increase local glucocorticoid signaling and contribute to whole-body cortisol regeneration (23). However, because the previous findings were not entirely consistent, it is still not clear whether the enzymatic overactivity is a cause or a result of obesity. In addition, our study is using a cross sectional design, and can therefore not assess whether cortisol levels actually contribute to weight gain over time. Hence, the present study can not test whether hypercortisolemia favors accumulation of visceral fat as initially suggested by Bjorntorp (4) and a recent review (24).
The present study showed that cortsiol levels were associated to fasting glucose levels in both men and women, even after controlling for age and BMI. This is in line with the notion that glucocorticoids stimulate hepatic gluconeogenesis, leading to increased fasting glucose (25). In addition, glucocorticoids inhibit insulin actions on skeletal muscle and potentiate actions on adipose tissue, promoting the development of the metabolic syndrome (26). Cortisol counteracts the insulin activation of glycogen synthesis in muscle, the insulin inhibition of hepatic glucose production and the insulin inhibition of lipolysis in adipose tissue, leading to systemic insulin resistance (27). Unfortunately, insulin was only available in a subsample of the present study participants. Our observation is somewhat consistent with in vivo and in vitro experiments showing that glucocorticoids regulate insulin secretion (28).
This differs from previous observations showing a synergistic effect of cortisol and adiposity on CVD risk factors. For example, studies have shown that the correlation between cortisol and systolic blood pressure is most marked in obese subjects (29). Although the mechanism of this interaction is unclear, a recent study suggests that glucocorticoid action may be increased in skeletal muscle, an important insulin target tissue, in obese individuals (30).
Our study population consisted of patients who visited a University Hospital, which may limit the generalizability of our findings. Second, because we employed a cross-sectional design, it is not possible to determine whether cortisol was causally related to the development of the metabolic syndrome. Longitudinal studies could assess whether circulating cortisol concentrations increases the risk of developing of the metabolic syndrome. Further research into the mechanisms behind these observations may lead to new therapeutic approaches to reduce the prevalence of diabetes and metabolic syndrome.
In summary, this study demonstrates that plasma cortisol concentration adds to the effect of adiposity on metabolic outcomes and suggests a relationship between HPA activity, as indicated by increased fasting cortisol concentration, and increased CVD risk independent of adiposity in Korean individuals.
Figures and Tables
Table 1
General characteristics of the study subjects
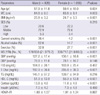
Values are mean ± SD unless otherwise indicated. BMI, body mass index; SES, socioeconomic status; MS, metabolic syndrome; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; T-Chol, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance. Gender difference of continuous variables were compared using t-test, categorical variables were compared by chi-squared test.
Table 2
Regression analysis of serum cortisol to CVD risk factors controlling for age and BMI in men and women

AUTHOR SUMMARY
Association of Cortisol and the Metabolic Syndrome in Korean Men and Women
Sat Byul Park, James A. Blumenthal, Soon Young Lee and Anastasia Georgiades
The cardiovascular disease (CVD) risk factors comprising the metabolic syndrome have previously been associated with increased hypothalamic-pituitary adrenal axis activity. The aim of the present study was to investigate the relationships between cortisol, adiposity and the metabolic syndrome in a Korean population (1,881 adults). Higher cortisol levels are associated with several CVD risk factors, independent of overall of adiposity level. In addition, there is an increased risk for the metabolic syndrome associated with higher cortisol levels after adjusting for age and BMI.
References
1. Pedersen SB, Jønler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994. 78:1354–1359.
2. Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, Fukushima Y, Onishi Y, Ono H, Fujishiro M, Kikuchi M, Oka Y, Asano T. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000. 49:1700–1708.
3. Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994. 35:1535–1541.
4. Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001. 2:73–86.
5. Morton NM. Obesity and corticosteroids: 11beta-Hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010. 316:154–164.
6. Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999. 83:25F–29F.
7. Meigs JB. Invited commentary: insulin resistance syndrome? Syndrome X? Multiple metabolic syndrome? A syndrome at all? Factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am J Epidemiol. 2000. 152:908–911.
8. Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001. 86:1418–1421.
9. Travison TG, O'Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf). 2007. 67:71–77.
10. Ward AM, Fall CH, Stein CE, Kumaran K, Veena SR, Wood PJ, Syddall HE, Phillips DI. Cortisol and the metabolic syndrome in South Asians. Clin Endocrinol (Oxf). 2003. 58:500–505.
11. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. 363:157–163.
12. McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991. 337:382–386.
13. Shelgikar KM, Hockaday TD, Yajnik CS. Central rather than generalized obesity is related to hyperglycaemia in Asian Indian subjects. Diabet Med. 1991. 8:712–717.
14. Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999. 84:137–144.
15. National Institutes of Health. Clinical Guidelines on the Identification, Evaluation and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998. 6:Suppl 2. 51S–209S.
16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.
17. Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet. 2005. 366:1059–1062.
18. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007. 75:72–80.
19. Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998. 83:1853–1859.
20. Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000. 62:623–632.
21. Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002. 106:2659–2665.
22. Salehi M, Ferenczi A, Zumoff B. Obesity and cortisol status. Horm Metab Res. 2005. 37:193–197.
23. Stimson RH, Andersson J, Andrew R, Redhead DN, Karpe F, Hayes PC, Olsson T, Walker BR. Cortisol release from adipose tissue by 11beta-hydroxysteroid dehydrogenase type 1 in humans. Diabetes. 2009. 58:46–53.
24. Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009. 9:787–793.
25. Barthel A, Scherbaum WA, Bornstein SR. Novel aspects in the mechanisms of steroid diabetes and the regulation of hepatic glucose production by insulin and steroids. Med Klin (Munich). 2003. 98:283–286.
26. Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000. 24:Suppl 2. S50–S55.
27. Björntorp P. Neuroendocrine perturbations as a cause of insulin resistance. Diabetes Metab Res Rev. 1999. 15:427–441.
28. Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997. 100:2094–2098.
29. Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from three populations. Hypertension. 2000. 35:1301–1306.
30. Whorwood CB, Donovan SJ, Flanagan D, Phillips DI, Byrne CD. Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes. 2002. 51:1066–1075.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download