Abstract
Thyroid carcinogenesis is accompanied by loss of thyroid-specific functions and refractory to radioiodine and thyroid stimulating hormone (TSH) suppression therapy. Redifferentiating agents have been shown to inhibit tumor growth and improve the response to conventional therapy. Polyphenol phytochemicals (PPs) in fruits and vegetables have been reported to inhibit cancer initiation, promotion, progression and induce redifferentiation in selected types. In this study we examined PPs induce redifferentiation in thyroid cancer cell lines. We investigated the effects of genistein, resveratrol, quercetin, kaempferol, and resorcinol on the F9 embryonal carcinoma cell differentiation model. The thyroid cancer cell lines, TPC-1, FTC-133, NPA, FRO, and ARO, displayed growth inhibition in response to genistein, resveratrol, quercetin. We further demonstrated that genistein decreased the dedifferention marker CD97 in NPA cells and resveratrol decreased CD97 in FTC-133, NPA, FRO cells and quercetin decreased CD97 in all cell lines. We observed increased expression of differentiation marker NIS in FTC-133 cells in response to genistein, and resveratrol but no change in NPA, FRO, ARO cells. Quercetin increased or induced NIS in FTC-133, NPA, FRO cells. These findings suggest that PPs may provide a useful therapeutic intervention in thyroid cancer redifferentiation therapy.
Differentiated thyroid cancers (DTC) maintain thyroid specific functions even with regional metastasis, respond to surgical treatment, radioiodine therapy and TSH suppression therapy. However, poorly differentiated (PDTC) or anaplastic thyroid cancers (ATC) do not respond to conventional therapy, leading to poor outcome (1). During the dedifferentiation process, the cancer loses thyroid-specific gene expression, including the ability to absorb radioiodine and to express the TSH receptor; as such radioiodine therapy or thyroid stimulating hormone (TSH) suppressive therapy is not effective in PDTC/ATC. Dedifferentiated cancers display more rapid growth properties and are refractory to chemotherapy, external beam radiation therapy, and show poor outcomes in comparison with differentiated cancers.
Redifferentiation therapy is defined as a treatment to inhibit tumor growth and regain thyroid-specific functions in PDTC and ATC as a mean of increasing the response to conventional therapy (2). The goals of such therapy are to regain the expression of differentiation markers, including the sodium iodine symporter (NIS), and to reduce levels of the dedifferentiation marker CD97. Plasma membrane expression of NIS in thyroid follicular cells is necessary to absorb iodine into the cell and, as such thyroid cancers with reduced NIS expression display impaired iodine uptake (3). CD97 is a dedifferentiation marker that is increased during cancer progression and metastasis, and is reduced by redifferentiating agent such as retinoid acid (RA) in partially dedifferentiated thyroid cancer cells (4).
Currently available redifferentiating agents include RA, histone deacetylase inhibitors (HDAC inhibitors), and peroxisome proliferator activated receptor gamma (PPARγ) agonist. Their effectiveness in dedifferentiated cancers and patient tolerability have been variable (1, 2, 5-7). Thus, this is a reason why more effective and better tolerated agents are needed.
The polyphenol phytochemicals (PPs) such as quercetin, resveratrol, genistein, and kaempferol (8-11), are present in fruits and vegetables, and inhibit cancer initiation, promotion and progression in vivo and in vitro. Moreover these compounds have been reported to induce redifferentiation in specific cancer types (12-14).
The F9 mouse embryonal carcinoma cell model (F9 cells) is frequently used to evaluate cellular morphological and molecular differentiation changes following drug and hormone treatment. The time duration of the growth cycle is very short and no self differentiation is evident; however, drugs including RA have been demonstrated to induce differentiation after 72 hr of treatment. The primitive endoderm-like structure is formed with the expression of laminin B1, collagen type IV, and tissue plasminogen activator (tPA), followed by growth inhibition (15).
In the present study, we tried to demonstrate that some PPs have the ability to induce antiproliferation and redifferentiation in thyroid cancer cell lines using the F9 embryonal carcinoma cell differentiation model.
F9 embryonal cancer, cells were cultured in DMEM (Sigma, St Louis, MO, USA) with 20% fetal bovine serum (FBS). TPC-1 (papillary thyroid cancer cell line) and FTC-133 (follicular thyroid cancer cell line), cells were cultured in DMEM with 10% FBS. NPA (poorly differentiated papillary thyroid cancer cell line), FRO (undifferentiated/anaplastic thyroid cancer cell line), and ARO (undifferentiated/anaplastic thyroid cancer cell line) cells were cultured in RPMI1640 (Sigma) with 10% FBS.
Polyphenol phytochemical (resveratrol, genistein, quercetin, kaempferol, resorcinol (Sigma, Fig. 1) were dissolved in dimethylsulfoxide (DMSO; Sigma) at appropriate concentrations prior to dilution in tissue-culture medium such that the final concentration of DMSO not exceed 0.1% (v/v). Each reagent was assessed for a concentrations-dependent effect in F9 and thyroid cancer cells. In F9 cells, 0.2 µM ATRA (all-trans-retinoic acid; Sigma) was used as a positive control.
Cell proliferation and growth inhibition was measured by colorimetric dimethyl-thiazol-diphenyltetrazolium bromide (MTT) proliferation assays.
At 72 hr after the administration of reagents, RT-PCR was performed for laminin B1, collagen type IV, and tissue plasminogen activator (tPA) mRNA in F9 cell. To assess thyroid cancer redifferentiation, RT-PCR was performed for NIS, CD97 mRNA; 36B4 mRNA was assessed as a housekeeping gene. RNA extraction and complementary DNA preparation were performed by conventional methods. The primers for each gene were designed and PCRs performed according to the following conditions (annealing temperature/cycles). 36B4: forward 5'-CAGCTCTGGAGAAACTGCTG-3', reverse 5'-GTGTACTCAGTCTCCACAGA-3' (49℃/25 cycles); laminin B1: forward 5'-TGAATTCTCACTGCAGACATGGTGAAG-3', reverse 5'-ATGGATCCCTCACTTATGTCCTTAAGGA-3' (48℃/30 cycles); collagen type IV: forward 5'-ATGAATTCTCAGCGTCTGGCTTCTGCT-3', reverse: 5'-ATGGATCCGTTGCATCCTGGGATACCTG-3' (62℃/30 cycles); tPA forward 5'-TTGGGCAGAACATACAGGGTGGTC-3', reverse 5'-CTGGCATGCATCGTGGAGGTCTT-3' (62℃/30 cycles); NIS forward 5'-GCCCTCATCCTGAACCAAGTG-3', reverse 5'-TGATCCGGGAGTGGTTCTG-3' (50℃/30 cycles); CD97 forward 5'-TCCTGCCGGCAGCTCCAACC-3', reverse 5'-GGCAGCGGCAGCTGTATGAAC-3' (58℃/30 cycles). PCR was conducted for the indicated number of cycles with denaturation at 95℃ for 60 sec, annealing at the temperatures indicated for 60 sec, and extension at 72℃ for 20 sec. Each RT-PCR experiment from F9 cell and thyroid cancer cells was performed in triplicate.
In thyroid cancer cell experiments, the PCR results were analyzed by semi quantitative methods using the Total Lab TL100 program (Nonlinear Dynamics Ltd., Newcastle, UK) compensated by comparing the 36B4 density of each PCR.
Statistical analyses were performed using the SPSS software (ver. 12.0 for Windows SPSS, Chicago, IL, USA). All MTT assays were performed in triplicate and mean value were compared using Student's t-test and one-way ANOVA. A P value < 0.05 was considered to indicate statistical significance.
We initially assessed the effects of resorcinol, genistein, resveratrol, kaempferol and quercetin on F9 cell proliferation at concentrations of 1, 10, 50, and 100 µM respectively. Fig. 2 shows that resorcinol did not inhibit growth at concentrations below 100 µM, while genistein, resveratrol, kaempferol and quercetin all inhibited cellular growth in a dose dependent manner. Following the administration of each agent, MTT assays were performed at 24, 48, 72, 96, and 120 hr. Fig. 3 demonstrates that F9 cell growth was increased in a time-dependent manner in control cells (DMSO only), while quercetin, resveratrol and genistein inhibited cell growth up to 120 hr (P < 0.05). Kaempferol did not affect F9 cell growth.
We thus selected quercetin, resveratrol and genistein for further experiments.
Genistein, resveratrol and quercetin at concentration of 10 µM and ATRA (positive control) at a concentration of 0.2 µM were administered to F9 cells in culture. After 72 hr, RT-PCR was performed for laminin B1, tPA and collagen type IV mRNA. Fig. 4 demonstrates that genistein, resveratrol and quercetin increased the levels of these differentiation markers compared with the DMSO-alone negative control.
Fig. 5 demonstrates that when genistein and quercetin were administered for 72 hr, the growth of TPC-1, NPA, FTC-133, FRO, and ARO cells was inhibited in a dose-dependent manner (P < 0.05). Resveratrol inhibited the growth of these cells from 10 µM excluding TPC-1 cells that displayed increased levels of growth. TPC-1 proliferation was, however, inhibited at resveratrol concentrations of 50 µM or more.
RT-PCR for CD97 and NIS mRNA was performed following 72 hr of treatment for TPC-1 cells: genistein (100 µM), resveratrol (50 µM), quercetin (100 µM), for NPA cells: genistein (20 µM), resveratrol (50 µM), qeurcetin (100 µM), for FTC-133 cells: genistein (20 µM), resveratrol (20 µM), quercetin (20 µM), for FRO cells: genistein (50 µM), resveratrol (50 µM), quercetin (20 µM), for ARO cells: genistein (50 µM), resveratrol (20 µM), quercetin (20 µM). In contrast to the other cell lines under investigation, TPC-1 cells did not express the dediffenetiation marker CD97. We observed a decrease in CD97 expression following genistein treatment in NPA cells. Resveratrol decreased CD97 in FTC-133, NPA and marginally decreased CD97 in FRO cells, while qurcetin decreased CD97 levels in FTC-133, NPA, FRO, and ARO cells. Expression of CD97 in TPC-1 cells was unaffected by the presence of any of the PPs (Fig. 6). NIS was expressed in TPC-1 and FTC-133 cells. While the levels of NIS were unchanged in the presence of genistein, resveratrol and quercetin in TPC-1 cells, all three compounds increased NIS levels in FTC-133 cells. Genistein and resveratrol both failed to induce in NPA, FRO or ARO cells. Qurcetin did not induce NIS in ARO cells but did induce NIS in dedifferentiated/anaplastic NPA and FRO cells (Fig. 7).
RA, HDAC inhibitor, and PPARγ agonists are effective redifferentiation therapies in partially dedifferentiated thyroid cancers, but are ineffective undifferentiated/anaplastic cancers and display high patient toxicity or effectiveness only in vitro. Polyphenol phytochemicals have been reported to inhibit cancer initiation, promotion and progression in vivo or in vitro and moreover have been reported to induce redifferentiation in specific cancer types (12-14). We present the first report that identifies the ability of PPs to promote tumor growth inhibition in both partially dedifferentiated cancer and dedifferentiated/anaplastic cancers.
We demonstrated that genistein (rich in soybeans), resveratrol (rich in grapes), quercetin (rich in onions) inhibit the tumor growth of TPC-1, FTC-133, NPA, FRO, and ARO cell lines. The mechanism of the growth inhibition of genistein, resveratrol and quercetin is thought to involve the inhibition of epidermal growth factor (EGF), NF-κB, cyclin D and protein tyrosine kinase, all of which increase tumor promotion, progression, and inreases caspase which can induce apoptosis (16-18). They have been tested in normal and cancer cultured cells, and shown that these compounds induced apoptosis in cultured cancer cells but not in their normal counterparts up to 100 µM, so these effects were not by general toxicity (19). Other studies have suggested that PPs act as phytoestrogens and inhibit the estrogen-dependent growth of estrogen receptor (ER) - positive cancer cells (20) and specific cancer cells (NPA, FRO) that express ER (11). We observed increased TPC-1 cell growth in response to low dose resveratrol (10 µM), most likely caused by its phytoestrogenic effects, stimulating the estrogen receptor α (ERα) and inhibitory estrogen receptor β (ERβ). The dose of resveratrol displays biphasic effects in MCF-7 breast cancer cells and low dose are thought to affect growth stimulation (21). However, no report has described the expression of ER in TPC-1 cells.
CD97 is a representative dedifferentiation marker in thyroid cancers that is stimulated by EGF, and can be inhibited genistein, resveratrol and quercetin (16), consistent with their effects on cancer cell observed in our study.
The dedifferentiation process is accompanied by both genetic mutations and epigenetic changes. During dedifferentiation process, a loss of NIS gene expression occurs, caused by hypermethylation of the NIS gene; this is recovered by demethylating agent 5-azacytidine (22). Additionally, NIS expression is induced by TSH mediated by TSH receptor (TSHR). Dedifferentiated thyroid cancers are accompanied by a loss of the TSHR gene promoter hypermethylation and is restored by 5-azacytidine (23). DNA methylation is mediated by DNA methyltransferases (DNMT) and numerous cancers including thyroid cancers, have been reported to have stimulated hypermethylation (22, 23). In this study, genistein, resveratrol and quercetin all incresed NIS expression in FTC-133 cells while quercetin also incresed NIS expression in dedifferentiated/anaplastic NPA and FRO cells. Quercetin has been reported to reverse DNA hypermethylation in a dose-dependent manner in other cancers (24). Genistein has been reported to reverse retinoic acid receptor β (RARβ) promoter hypermethylation and increase RARβ in prostate and esophageal cancers (25). There is no report regarding the effects of resveratrol in relation to DNA methylation; however, most of PPs are substrates of catechol O-methyltransferase (COMT) and are converted to S-adenosyl-L-homocysteine (SAH) by COMT, an antagonist of DMNT (24). Additionally, resveratrol has been reported to increase the transcription of PPARγ, which can induce thyroid cancer redifferentiation (26). RA acts through binding with RARβ and has been reported to restore NIS expression in FTC-133 (27), but not in NPA, FRO or ARO cells. Additionaly RA does not inhibit growth of FRO and ARO cells that have lost RARβ (28), which occurs as a result of promoter hypermethylation in numerous thyroid cancer cell lines (29). Thus, PPs that inhibit RARβ hypermethylation, when administered in combination with low-dose RA, may be effective in thyroid cancers that are refractory to RA therapy. Serum concentrations of PPs typically do not exceed 10 µM in humans, a concentration that is both antiproliferative and induces redifferentiative in vitro. The tissue concentration levels of these compounds can increase 1-10 fold following dietary supplementation (30).
In conclusion these findings suggest that genistein, resveratrol and quercetin may be effective for thyroid cancer redifferentiation therapy, including of undifferentiated/anaplastic cancers. Further investigations are required to increase the tissue concentrations of these polyphenol phytochemicals in thyroid cancer patients and to assess their effectiveness in patients with dedifferentiated thyroid cancers.
Figures and Tables
 | Fig. 2F9 cell MTT assays in the presence of each polyphenol phytochemicals. Results are the mean ± SD of triplicate experiments. P values were determined by comparison with the control samples using Student's t- test. (A) F9 cell MTT assays in the presence of resorcinol, (B) F9 cell MTT assays in the presence of genistein, (C) F9 cell MTT assays in the presence of resveratrol, (D) F9 cell MTT assays in the presence of kaempferol, (E) F9 cell MTT assays in the presence of quercetin. |
 | Fig. 3F9 cell MTT assays in the presence of each polyphenol (10 µM). Results are the mean ± SD of triplicate experiments. P values were determined by comparison with the control samples using Student's t-test. *P = 0.001; †P < 0.001; ‡P = 0.001; §P < 0.001. |
 | Fig. 4F9 cell differentiation marker expression in the presence of ATRA and the polyphenol phytochemicals. Representative results are shown. 1, DMSO; 2, ATRA 0.2 µM; 3, Genistein 10 µM; Resveratrol 10 µM; Quercetin 10 µM. |
 | Fig. 5MTT assays in thyroid cancer cell lines in response to polyphenol phytochemicals. Results are shown as the mean ± SD of triplicate esperiments. *,†P values were determined in comparison with the control using Student's t-test. a, b, c, d, e: P values were determined using one way ANOVA. (A) MTT assays in thyroid cancer cell lines in response to resveratrol. *,†P < 0.01: a, b, c, d, e; P < 0.001. (B) MTT assays in thyroid cancer cell lines in response to genistein; *P < 0.05, †P < 0.01: a, b, c ,d, e; P < 0.001. (C) MTT assays in thyroid cancer cell lines in response to quercetin. *P > 0.05, †P < 0.05: a, b, c ,d, e; P < 0.001. |
 | Fig. 6RT-PCR for CD97 mRNA in thyroid cancer cell lines. (A) RT-PCR for CD97 mRNA levels in thyroid cancer cell lines following genistein, resveratrol or quercetin treatment (1, Control; 2, Genistein 100 µM; 3, Resveratrol 50 µM; 4, Quercetin 100 µM; 5, Control; 6, Genistein 20 µM; 7, Resveratrol 50 µM; 8, Quercetin 100 µM; 9, Control; 10, Genistien 20 µM; 11, Resveratrol 20 µM; 12, Quercetin 20 µM; 13, Control; 14, Genistein 50 µM; 15, Resveratrol 50 µM; 16, Quercetin 20 µM; 17, Control; 18, Genistein 50 µM; 19, Resveratrol 20 µM; 20, Quercetin 20 µM). (B) Relative CD97 density in thyroid cancer cell lines following genistein, resveratrol or quercetin treatment. Results are normalized to the relative density of 36B4. |
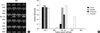 | Fig. 7RT-PCR for NIS mRNA in thyroid cancer cell lines. (A) RT-PCR for NIS mRNA in thyroid cancer cell lines following genistein, resveratrol or quercetin treatment (1, Control; 2, Genistein 100 µM; 3, Resveratrol 50 µM; 4, Quercetin 100 µM; 5, Control; 6, Genistein 20 µM; 7, Resveratrol 50 µM; 8, Quercetin 100 µM; 9, Control; 10, Genistien 20 µM; 11, Resveratrol 20 µM; 12, Quercetin 20 µM; 13, Control; 14, Genistein 50 µM; 15, Resveratrol 50 µM; 16, Quercetin 20 µM; 17, Control; 18, Genistein 50 µM; 19, Resveratrol 20 µM; 20, Quercetin 20 µM). (B) Relative NIS density in thyroid cancer cell lines following genistein, resveratrol or quercetin treatment. Results are normalizd to the relative density of 36B4. |
ACKNOWLEDGMENTS
We thank the senior investigator Drs. Kuk Jin Choe, Bo Youn Cho, Sung-Eun Jung and Do-Joon Park for their help throughout the study.
AUTHOR SUMMARY
Antiproliferation and Redifferentiation in Thyroid Cancer Cell Lines by Polyphenol Phytochemicals
Hee Joon Kang, Yeo-Kyu Youn, Mi-Kyoung Hong and Lee Su Kim
This study assessed the ability of polyphenol phytochemicals (PPs) as a redifferentiatiion therapy in thyroid cancer cells. Using F9 cell differentiation model for the selection of effective PPs, we demonstrated that genistein, resveratrol, quercetin were effective. In five kinds of thyroid cancer cell lines, we further demonstrated that the PPs not only decrease the dedifferention markers, but also increase the expression of differentiation marker. These findings suggest that the PPs investigated in this study might be considered for the redfferentiation therapy of thyroid cancer.
References
1. Park JW, Clark OH. Redifferentiation therapy for thyroid cancer. Surg Clin North Am. 2004. 84:921–943.
2. Schmutzler C, Koehrle J. Innovative strategies for treatment of thyroid cancer. Eur J Endocrinol. 2000. 143:15–24.
3. Lazar V, Bidart JM, Caillou B, Mahé C, Lacroix L, Fletti S, Schlumberger M. Expression of the Na+/I- symporter gene in human thyroid tumors: a comparison study with other thyroid specific genes. J Clin Endocrinol Metab. 1999. 84:3228–3234.
4. Hoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, Köhrle J, Dralle H. Regulation of CD97 protein in thyroid carcinoma. J Clin Endocrinol Metab. 1999. 84:1104–1109.
5. Shen WI, Chung WY. Treatment of thyroid cancer with histone deacetylase inhibitor and peroxisome proliferater-activated receptor-γ agonists. Thyroid. 2005. 15:594–599.
6. Zarnegar R, Brunaud L, Kanauchi H, Wong M, Fung M, Ginzinger D, Duh QA, Clark OH. Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. Surgery. 2002. 132:984–990.
7. Park JC, Ryu DH, Park JW, Clark OH. Redifferentiation effects of troglitazone in human thyroid cancer cell lines. J Korean Surg Soc. 2004. 67:93–99.
8. Chen YC, Shen SC, Chow JM, Ko CH, Tseng SW. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. Int J Oncol. 2004. 25:661–670.
9. Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee TS, Lee SJ. Induction of cell cycle arrest and apotosis in human breast cancer cells by quercetin. Int J Oncol. 2001. 19:837–844.
10. Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005. 81:1 Suppl. 284S–291S.
11. Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell line. Thyroid. 1999. 9:369–376.
12. Asou H, Koshizuka K, Kyo T, Takata N, Kamada N, Koeffier HP. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias. Int J Hematol. 2002. 75:528–533.
13. Dihal AA, Woutersen RA, van Ommen B, Rietjens IM, Stierum RH. Modulatory effects of quercetin on proliferation and differentiation of the human colorectal cell line Caco-2. Cancer Lett. 2006. 238:248–259.
14. Nakamura Y, Chang CC, Mori T, Sato K, Ohtsuki K, Upham BL, Trosko JE. Augmentation of diffentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis. 2005. 26:665–671.
15. Alonso A, Breuer B, Steuer B, Fisher J. The F9-EC line as a model for the analysis of differentiation. Int J Dev Biol. 1991. 35:389–397.
16. Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006. 71:1397–1421.
17. Gulati N, Laudet B, Zohravian VM, Murali R, Jhanwa-Uniyal M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006. 26:1177–1181.
18. Mertens-Talcott SU, Bomser JA, Romero C, Talcott ST, Percival SS. Ellagic acid potentiates the effect of quercerin on p21waf1/cip1, p53, and MAP-kinase without affecting intracellular generation of reactive oxygen species in vitro. J Nutr. 2005. 135:609–614.
19. Ramos S. Effect of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007. 18:427–442.
20. Folman Y, Pope GS. The interaction in the immature mouse of potent oestrogens with coumestrol, genistein and other utero-vaginotrophic compounds of low potency. J Endocrinol. 1966. 34:215–225.
21. Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alph- or beta mediated response in transfected breast cancer cells. Exp Biol Med (Maywood). 2005. 230:558–568.
22. Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodine uptake in dedifferentiated thyroid carcinoma: relatioship to human Na+/I- symporter gene methylation status. J Clin Endocrinol Metab. 1999. 84:2449–2457.
23. Das PM, Singal R. DNA methyation and cancer. J Clin Oncol. 2004. 22:4632–4642.
24. Lee WJ, Shim JY, Zhu BT. Mechanism for the inhibiton of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005. 68:1018–1030.
25. Fang MZ, Wang Y, Ai N, Hou AZ, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003. 63:7563–7570.
26. Ulrich S, Loitsch SM, Rau O, von Knethen A, Brüne B, Schubert-Zsilavecz M, Stein JM. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006. 66:7348–7354.
27. Schmutzler C, Köehrle J. Retinoic acid redifferentiation therapy for thyroid cancer. Thyroid. 2000. 10:393–406.
28. Elisei R, Vivaldi A, Agate L, Ciampi R, Monlinaro E, Piampiani P, Romei C, Favaiana P, Basolo F, Miccoli P, Capodano A, Collecchi P, Pacini F, Pinchera A. All-trans-Retinoic acid treatment inhibits the growth of retinoic acid receptor β messenger ribonucleic acid expressing thyroid cancer cell lines but does not reinduce the expression of thyroid-specific genes. J Clin Endocrinol Metab. 2005. 90:2403–2411.
29. Hoque MO, Rosenbaum E, Westra WH, Xing M, Ladenson P, Zeiger MA, Sidransky D, Umbricht CB. Quantitative assessment of promoter methylation profiles in thyroid neoplasm. J Clin Endocrinol Metab. 2005. 90:4011–4018.
30. Gardner CD, Jenny B, Liu JP, Feldman D, Franke AA, Brooks JD. Prostate soy isoflavone concentrations exceed serum levels after dietary supplementation. Prostate. 2009. 69:719–726.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download