Abstract
Phantom limbs are usually observed after amputation of extremities. In patients after a stroke, a similar but rarely occurring phenomenon consisting of the patient experiencing the presence of an additional limb has been described. This phenomenon, generally called supernumerary phantom limb (SPL), may be caused by lesions in the right or left cerebral hemisphere, but has been predominantly reported in patients who have had a right hemispheric stroke. We report two cases of atypical SPL and phantom limb pain (PLP) after pontine hemorrhage. The patients were treated conservatively and their symptoms lasted more than 1 month. This is the first report of SPLs after left pontine hemorrhage, and phantom perception and pain lasted longer than those in previously observed cases. Our results indicate that SPL may be more common than reported; therefore, thorough examinations are essential for the care of stroke patients.
Patients with brain damage may be unaware of the deficits that contribute to their impaired performance in activities of daily living (1). This may be because of a lack of awareness of the involved limb (anosognosia) (2), the limb acting on its own and lacking voluntary control (alien hand syndrome) (3), and the feeling of having an extra limb (supernumerary phantom limb or SPL) (4). The term 'phantom limb' (PL) is used to denote the syndrome in which a patient experiences the vivid illusion that an amputated extremity is still present. The phantom limb phenomenon has been reported not only in amputees, but also in patients with deafferentation due to lesions at various levels of the neuraxis (5). PL arising from nervous system damage, not amputation, was termed SPL or phantom third limb to denote "the illusory feeling of a limb in an attitude which does not conform to the actual posture of the paralyzed limb" (4, 6). SPL resulting from cerebral lesions are considered to be rare (7). Miyazawa et al. (8) reported only 20 cases of SPL resulting from stroke or cerebral hemorrhage in the last 70 yr. SPL may be caused by lesions in the right or left cerebral hemisphere (9) but has been reported more frequently after right hemispheric stroke (10). No localized brain lesions for SPL and phantom limb pain (PLP) have been clearly established nor has SPL with PLP after left pontine hemorrhage been reported. We now report two atypical cases of SPL with PLP in two patients with pontine hemorrhage.
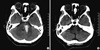
A 53-yr-old right-handed woman who was a housewife was hospitalized in our department on December 30, 2008 after presenting an altered mental state. Her medical history included hypertension, coronary heart disease, and an old lacunar infarction. A neurological examination revealed consciousness disturbances, motor aphasia, right hemiparesis, superficial and deep sensory disturbances, and right-side neglect. Computed tomography (CT) scans obtained at the time of admission revealed an area of left pontine hemorrhage measuring 1.0 × 2.5 cm (Fig. 1A). Two months after admission, CT scans demonstrated that the high-density area in the left pons had completely disappeared (Fig. 1B), and the patient scored 25 out of 30 on Mini-Mental State Examination (MMSE). Her score on the NIH Stroke Scale (NIHSS) was 11, and 24 on the Modified Barthel Index (MBI). She had hemianopia, left facial palsy, and right hemiparesis showing grade poor on manual muscle test (MMT) for right upper limb and grade fair on MMT for right lower limb, and a visual analogue scale (VAS) result of 50 mm for the right limbs with paresthesia. She was totally dependent on others for daily living tasks because of right side weakness. At that time, she was able to name her body parts correctly, and there was no evidence of other delusional beliefs or hallucinations.
The patient repeatedly started to report that she felt the presence of another set of right upper and lower extremities protruding from her right shoulder and knee. When asked about how she knew about these arm and leg on February 25, 2009, she said that she could not see them but feel them with her extremities, and could draw her phantom limbs (Fig. 2). She believed that the phantom limbs did not belonged to her, and did not know why she had phantom limbs. She did not have pain on her original extremities, but complained of a squeezing and burning pain in her SPLs, and a VAS of the phantom pain was 80 mm. The SPLs started to be felt on a daily basis and she could not move her SPLs voluntarily. Her PLP increased during night while lying in bed, and she received occupational, cognitive therapy and medical treatment. She was started on 300 mg gabapentin per day, and increased the dose by 300 mg per day every 3-4 weeks. She did not respond to a 3-month treatment with 1,200 mg of gabapentin, and the VAS result was 70 mm on May 26. Her condition gradually improved by changing to a course of high-dose pregabalin (lyrica® 150 mg per day), the VAS was 50 mm on June 22, and 30 mm on July 20. Her belief of having a third leg and associated PLP disappeared on August 17. The belief of having a third arm with associated PLP persisted throughout her year-long stay in hospital, and her symptom was relieved on April, 2010.
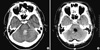
A 51-yr-old right-handed man was hospitalized on August 27, 2009 because of a sudden onset headache and diplopia, and was admitted under neurological intensive care at another hospital during the acute phase. At that time, he had right hemiplegia, diplopia, and elevated blood pressure. There was no history of diabetes, hypertension, epilepsy, psychiatric or neurological illness. Initial CT scans at the time of admission revealed intracerebral hemorrhage involving the left pons (Fig. 3A). About 20 days after the onset of weakness, his symptoms persisted and underwent hematoma evacuation on September 15, 2009 (Fig. 3B).
The patient was admitted to our department on January 6, 2010 because of residual weakness, paresthesia in right side of body. A neurological examination at admission revealed hemianopia, right hemiparesis with grade fair on MMT in right upper limb and grade good on MMT in right lower limb, and superficial and deep sensory disturbances with 60 mm on VAS in right upper limb and 30 mm on VAS in right lower limb. He scored 28 out of 30 on MMSE which indicated below average orientation and memory recall. His score on the NIHSS was 7, and 45 on the MBI.
The patient started making remarks about a paralysed right upper limb on January 13, 2010. He noted that 'the other limb is attached to my right shoulder, and this is not my limb', complained daily about tingling sensations and a burning pain both in his original arm and phantom limb, with VAS was of 50 mm and 70 mm. He also noted that his phantom arm never moved spontaneously, and when the right arm was passively moved, the SPL also moved. He could feel the SPL but not visualize the movements of the SPL. Pregabalin (lyrica®) was started with a bedtime dose of 75 mg, and increased to 150 mg per day a week later. His symptoms gradually improved with a course of high-dose pregabalin along with occupational and cognitive therapy, the VAS was 30 mm and 50 mm on January 27, and the VAS was 10 mm and 20 mm on February 9. He was discharged from hospital on February 11, and the belief in his SPL and PLP disappeared at the 1-month follow-up examination on March 17.
SPL highlights the brain mechanisms of consciousness (11). The hypotheses for the cause of this phenomena range from psychological to organic. However, previous studies have shown that PLP is not a function of emotional adjustment (12). Halligan et al. (13) and Halligan and Marshall (14) have reported normal cognition and psychological profiles in patients with SPL. This suggests a possible organic cause for the reported SPL. The patients reported here had the classic features of SPL, and persistent burning PLP. The phantom perception was vivid, recurrent, and stable (i.e., the description did not change over time). The patients were mentally alert with good cognitive function, and there was no lack of insight for neurological deficit or its consequences.
Most cases of SPL are accompanied to a variable degree by both sensory and motor impairment. The incidence of SPL may be determined more by motor than sensory loss (10, 13). Therefore, spastic hemiparesis may be involved in SPL. The anatomical and functional integrity of the non-dominant parietal lobe and other brain structures and sensory feedback from the peripheral nervous system seem essential for bodily awareness (body schema). Disruption of any of these structures may result in the perception of phantom sensations (5). SPLs were first reported in patients with parietal lobe lesions (4). However, it subsequently became clear that other brain areas, including the thalamus (8), supplementary motor area (15), and motor cortex (10) contribute to the conscious perception of body schema.
The reason why SPL phenomenon is more commonly associated with right rather than left hemispheric stroke is still unclear. The right hemisphere appears to be involved in monitoring somatic states with a primary role in processing somatic representation (11), and is dominant in maintaining the internal representation of the body state (10). The attentional network may also be controlled by the right hemisphere (16). Furthermore, the incidence of right thalamic hemorrhage is significantly higher than that of left thalamic hemorrhage (17).
Previous cases of SPL on the right side of the body involved no precise description of sensory disturbances (18) or sensory disturbances less severe than anesthesia (19). In our case, severe anesthesia to superficial and profound stimuli was present; therefore, whether any sensory disturbance is characteristic of SPL remains unknown. The SPLs in our case illustrates some possible causative factors of SPL among patients with left hemispheric stroke, including lesions located in the pons. This is the first report of SPLs after left pontine hemorrhage, and the SPL and PLP lasted longer than cases described in past studies (1, 6, 9, 13). SPLs can be underreported that detailed exploration into body image is not part of standard clinical assessment. In addition, the perceptions reported were not only brief and transient but also often regarded by many of the participants as inconsequential amidst the overwhelming clinical realities of stroke (20).
Figures and Tables
Fig. 1
Initial and follow-up computed tomography of the brain. (A) CT scans of the brain at the time of the first admission showed the left pontine area of hemorrhage measuring 1.0 × 2.5 cm. (B) CT scans of the brain at 2 months after the first admission showed encephalomalatic change of the left pontine area.
References
1. Srivastava A, Taly AB, Gupta A, Murali T, Noone ML, Thirthahalli J, Gangadhar BN, Kumar JK, Jayakumar PN. Stroke with supernumerary phantom limb: case study, review of literature and pathogenesis. Acta Neuropsychiatr. 2008. 20:256–264.
2. McGlynn SM, Schacter DL. Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol. 1989. 11:143–205.
3. Brion S, Jedynak CP. Disorders of interhemispheric transfer (callosal disonnection). 3 cases of tumor of the corpus callosum. The strange hand sign. Rev Neurol (Paris). 1972. 126:257–266.
4. Critichley M. The parietal lobes. 1953. New York: Hafner;243–244.
5. Melzack R. Phantom limbs and the concept of a neuromatrix. Trends Neurosci. 1990. 13:88–92.
6. Fredericks J. FREDERICKS J, editor. Phantom limb and phantom limb pain. Handbook of clinical neurology. 1985. Vol. 1. Amsterdam: Elsiever;373–393.
7. Cutting J. Reynolds E, Trimble M, editors. Body image disturbances in neuropsychiatry. The bridge between neurology and psychiatry. 1989. Edinburgh: Churchill Livingstone.
8. Miyazawa N, Hayashi M, Komiya K, Akiyama I. Supernumerary phantom limbs associated with left hemisphere stroke: case report and review of the literature. Neurosurgery. 2004. 54:228–231.
9. Frederiks JA. Occurrence and nature of phantom limb phenomena following amputation of body parts and following lesions of the central and peripheral nervous system. Psychiatr Neurol Neurochir. 1963. 66:73–97.
10. Canavero S, Bonicalzi V, Castellano G, Perozzo P, Massa-Micon B. Painful supernumerary phantom arm following motor cortex stimulation for central poststroke pain. Case report. J Neurosurg. 1999. 91:121–123.
11. Canavero S. Dynamic reverberation. A unified mechanism for central and phantom pain. Med Hypotheses. 1994. 42:203–207.
12. Fisher K, Hanspal RS. Phantom pain, anxiety, depression, and their relation in consecutive patients with amputated limbs: case reports. BMJ. 1998. 316:903–904.
13. Halligan PW, Marshall JC, Wade DT. Three arms: a case study of supernumerary phantom limb after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 1993. 56:159–166.
14. Halligan PW, Marshall JC. Supernumerary phantom limb after right hemispheric stroke. J Neurol Neurosurg Psychiatry. 1995. 59:341–342.
15. McGonigle DJ, Hänninen R, Salenius S, Hari R, Frackowiak RS, Frith CD. Whose arm is it anyway? An fMRI case study of supernumerary phantom limb. Brain. 2002. 125:1265–1274.
16. Donnet A, Schmitt A, Poncet M, Graziani N, Grisoli F. Hallucinations of supernumerary limbs, left hemineglect and hypersexuality in a case of right capsulo-lenticular hematoma. Rev Neurol (Paris). 1997. 153:587–590.
17. Nasreddine ZS, Saver JL. Pain after thalamic stroke: right diencephalic predominance and clinical features in 180 patients. Neurology. 1997. 48:1196–1199.
18. Bogaert LV. Sur la pathologie de l'image de soi. Ann Med Psychol. 1934. 92:519–536.
19. Pinéas H. Ein Fall von phantomähnlichen Erscheinungen ("Phantomarm") bei hemiplegischer Lähmung. Nervenarzt. 1932. 5:233–236.
20. Antoniello D, Kluger BM, Sahlein DH, Heilman KM. Phantom limb after stroke: an underreported phenomenon. Cortex. 2009. 46:1114–1122.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download