Abstract
The aim of this study was to evaluate the efficacy of levofloxacin and rifaximin based quadruple regimen as first-line treatment for Helicobacter pylori infection. A prospectively randomized, double-blinded, parallel group, comparative study was performed. Three hundred consecutive H. pylori positive patients were randomized to receive: omeprazole, amoxicillin, clarithromycin (OAC); omeprazole, amoxicillin, levofloxacin (OAL); and omeprazole, amoxicillin, levofloxacin, rifaximin (OAL-R). The eradication rates in the intention to treat (ITT) and per protocol (PP) analyses were: OAC, 77.8% and 85.6%; OAL, 65.3% and 73.6%; and OAL-R, 74.5% and 80.2%. The eradication rate achieved with OAC was higher than with OAL on the ITT (P = 0.05) and PP analysis (P = 0.04). OAL-R regimen was not inferior to OAC. The frequency of moderate to severe adverse effects was significantly higher in OAC treatment group. Especially, diarrhea was most common complaint, and there was a significantly low rate of moderate to severe diarrhea with the rifaximin containing regimen. In conclusion, the levofloxacin and rifaximin based regimen comes up to the standard triple therapy, but has a limited efficacy in a Korean cohort. The rifaximin containing regimen has a very high safety profile for H. pylori eradication therapy.
Helicobacter pylori is a Gram-negative bacteria that colonizes the human stomach and plays a major role in peptic ulcer disease, low-grade mucosa-associated lymphoid tissue lymphoma and gastric cancer. Eradication of H. pylori is the recommended treatment for these conditions (1). Proton pump inhibitor (PPI) based triple therapy, which includes PPI, amoxicillin and clarithromycin/metronidazole is generally accepted as first-line therapy (2); however, there has been a substantial decline in the H. pylori eradication rates recently. The outcomes of current H. pylori eradication regimens have been disappointing (3). The reasons for eradication failure are diverse and they include antibiotic resistance, poor compliance, the short duration of therapy, drug-related adverse effects, bacterial load, smoking, and underlying disease (4, 5). Among them, antibiotic resistance is an important factor in the treatment failure; primary resistance to clarithromycin or metronidazole significantly affects the efficacy of eradication therapy (6, 7).
Rifaximin is an oral antibiotic that belongs to the rifabutin family. It is not absorbed by the gastric and intestinal mucosa and is highly concentrated in the gastrointestinal tract. It has a broad-spectrum of in vitro activity against Gram-positive or Gram-negative enteric bacteria (8). The minimum inhibitory concentration (MIC) of rifaximin is comparable to many antibiotics used for H. pylori eradication (9, 10). Because it is not absorbed, rifaximin has a low risk of causing bacterial resistance. Levofloxacin is a fluoroquinolone that exerts broad-spectrum bactericidal effects by inhibiting DNA gyrase. It is active against H. pylori in vitro and has a synergistic effect with PPIs (11). The currently reported efficacy of levofloxacin-based therapy for H. pylori eradication ranged from 60% to 90%, in per protocol (PP) analysis (12, 13).
The goal of this study was to determine the efficacy of levofloxacin and to evaluate the efficacy of levofloxacin and rifaximin based quadruple therapy as first line of treatment for H. pylori infection in a Korean cohort. In addition, the combination of rifaximin was studied for its synergistic effects for H. pylori eradication.
A prospectively randomized, double-blinded, parallel group, comparative study was performed between July 2008 and September 2009 at St. Vincent's Hospital, the Catholic University of Korea, Suwon.
All patients referred to the endoscopy unit were recruited for this prospective study. They were eligible to enter the study if they were older than 18 yr of age and had gastric H. pylori infection. The diagnosis of H. pylori infection was made based on 1) positive rapid urease test (CLOtest; Delta West, Bentley, Australia) or 2) histologic evidence of H. pylori in any of two specimens taken from antrum/corpus by silver stain. Patients were not permitted to take H2 receptor antagonists or any other ulcer healing drugs during the study period. None of the patients had a history of H. pylori eradication, previous gastric surgery or had taken antibiotics in the two months before the study. Patients were also excluded if they had significant renal, hepatic, cardiovascular, metabolic or hematological disorders. Additionally, pregnant or lactating women were excluded from our investigation.
An estimated sample size of 88 subjects per group would give an 80% power to detect a difference of 15% for the eradication rate in the levofloxacin-based therapy compared to the standard triple therapy (assumed to have an eradication rate of 80%), with a two-sided alpha = 0.05. With a 10% drop out rate we have to recruit at least 97 patients for each group.
All patients were randomized to receive one of the three first-line treatment regimens, all given for seven days. Randomization codes (A, B, C) were packed into sealed opaque envelopes by an individual not involved in screening and enrolment of subjects to ensure concealment of allocation. One pharmacist had a responsibility for dispensing the trial drugs to the patient based on the unique randomization code. The trial drugs were labelled by the manufacturer (A-1 to A-100, B-1 to B-100, C-1 to C-100). At the end of allocation, the pharmacist provided us with a randomization list.
Each group contained 100 eligible patients and received a different treatment regimen. Patients in group 1 received omeprazole 20 mg, clarithromycin 500 mg and amoxicillin 1.0 g, twice a day for 7 days (OCA); in group 2, omeprazole 20 mg, amoxicillin 1.0 g and levofloxacin 200 mg, twice a day for 7 days (OAL); and in group 3, omeprazole 20 mg, amoxicillin 1.0 g, levofloxacin 200 mg and rifaximin 400 mg, twice a day for 7 days (OAL-R). No further treatment was given, and instructions about adherence to treatment were given to each patient.
Eradication of H. pylori was assessed by the 13C-UBT at six weeks after completion of treatment. Proton pump inhibitors and antimicrobial agents that might affect the 13C-UBT were not given to the patients after completion of therapy. The 13C-UBT was performed with a capsule-based modification. In brief, the patients fasted for over 12 hr before examination and then a gelatin capsule containing 38 mg 13C-urea was ingested with 50 mL water. Breath samples before and 20 min after administration of 13C-urea were collected after a mouthwash. The 13C/12C ratio in the breath samples was measured by mass spectrometry (Heliview; MediChems, Seoul, Korea). The changes in the 13C value over the baseline were expressed as Δ13C. A positive result was defined as an increase of > 2‰.
The eradication rate was evaluated for each drug regimen by the per-protocol (PP) analysis based on the number of patients that completed the study, and by an intention-to-treat (ITT) analysis that took into account all of the patients, including those who dropped out because of severe adverse effects, those who had poor drug compliance, and those who were lost to follow-up. The patients were interviewed during their first visit to the clinic after completion of therapy to clarify their compliance with the therapy and identify any adverse effects. Poor compliance was defined as taking less than 90% of the total medication prescribed.
The questionnaire contained questions regarding personal history of smoking and alcohol drinking. Smokers were those who consumed more than one pack of cigarettes a week and drinkers were those who drank more than one cup of alcoholic beverage a day. All "adverse effects" were assessed by the questionnaire. The adverse events included anorexia, nausea, taste disturbance, dizziness, abdominal pain, diarrhea, headache, and skin eruption. For the purpose of analyses, adverse effects was considered as a binomial variable (present-absent) and scored as mild, moderate or severe according to their influence on the daily activities of living.
All data were recorded on standard forms and computer analyzed. The Student t-test was used to compare continuous variables between the groups. Differences between dichotomous variables were evaluated with the chi square test. The calculations were performed with the SPSS software (SPSS version 12.0, Chicago, IL, USA). P values less than 0.05 were considered significant.
The characteristics of all patients are summarized in Table 1. Five out of 300 patients were excluded because of the history of H. pylori eradication or recent use of antibiotics (n = 1 in OAC; n = 2 in OAL; n = 2 in OAL-R). Eleven patients discontinued treatment because of adverse effects (n = 5 in OAC; n = 3 in OAL; n = 3 in OAL-R). Follow-up was incomplete in 16 patients (n = 4 in OAC; n = 8 in OAL; n = 4 in OAL-R). The reasons for incomplete follow-up were emigration, private business or reluctance to participate in the clinical trial (Fig. 1).
H. pylori eradication (intention to treat) was successful in 77/99 (77.8%) with OAC, 64/98 (65.3%) with OAL and 73/98 (74.5%) with OAL-R. Per protocol analyses were: OAC, 77/90 (85.6%); OAL, 64/87 (73.6%) and OAL-R, 73/91 (80.2%). The eradication rate achieved with the OAL regimen was significantly lower than with the standard OAC regimen in the ITT (P = 0.05) and PP analysis (P = 0.04). The OAL-R regimen was not inferior to the OAC regimen (Fig. 2). No differences were found between the OAL and the OAL-R regimen (ITT, P = 0.16; PP, P = 0.29).
No significant differences in patient compliance and frequency of adverse effects were found between the groups. The frequency of moderate to severe adverse effects was significantly higher with the OAC regimen (Table 2). Diarrhea was the most common complaint. Moderate to severe diarrhea was significantly low with the OAL-R regimen (Fig. 3). Compliance with the therapy was good for all three regimens; however, 11 patients discontinued the treatment. Overall, 11 (1.7%) patients complained of diverse adverse effects, including diarrhea (n = 3), abdominal pain (n = 1), nausea (n = 2), a bitter taste (n = 1), skin rash/itching (n = 2), headache (n = 1) and facial edema (n = 1).
Over the past 20 yr, standard triple therapy for H. pylori infection has been adopted worldwide and recommended as first-line therapy by the Maastricht I and II consensus report (14, 15). However, it has limited efficacy, because of adverse effects, poor compliance of patients and the development of antibiotic resistance. Especially, primary resistance to metronidazole or clarithromycin significantly affects the efficacy of eradication therapy (16). Resistance to metronidazole is related to prior antibiotic exposure, but it does not always lead to treatment failure (17). However, primary clarithromycin resistance, which shows regional difference, is considered as a strong predictive risk factor for treatment failure (18). The recent Maastricht III consensus report recommended that the clarithromycin should not be used or susceptibility test should be performed where the threshold of this antibiotic was 15%-20% (19).
The risk for clarithromycin resistance was associated with previous treatment with macrolides (erythromycin and azythromycin) that induced cross resistance to clarithromycin (20). In clinical practice, physicians usually prescribe such antibiotics for respiratory infections and they are widely used for H. pylori eradication treatment. For example, in France where macrolides have been widely used for a long time, the initial rate of H. pylori strains resistant to clarithromycin was about 10% (21); however, it has increased up to 29.1% (22). Moreover, a recent study reported that the number of strains resistant to clarithromycin and metronidazole had gradually increased (23). In Korea, the clarithromycin resistance rate was reported to be below 5%; however, this rate increased gradually (24, 25). Now, the resistance rate for clarithromycin was reported to be 17.2% (MIC of >1.0 µg/mL) (26). For this reason, success rate of standard triple therapy is lowered in Korea.
To reduce antibiotic resistance of H. pylori, the use of antibiotics needs to be limited and susceptibility testing performed before initiation of the eradication therapy. The role of antibiotic sensitivity testing prior to starting first-line therapy has been controversial. There are conflicting results on whether antibiotic sensitivity testing would be an efficient approach to H. pylori eradication therapy (26, 27). Therefore, choosing eradication regimens other than the standard triple therapy, as first-line therapy, might be advisable in areas with a high prevalence of primary antibiotic resistance. Rifaximin was reported to be active against H. pylori strains resistant to clarithromycin (9); thus suggesting that it might be useful in combination with this drug. Moreover, it has a low risk of causing antibiotic resistance, and rifaximin can be a good candidate for H. pylori eradication.
This study was conducted to assess the efficacy of levofloxacin and rifaximin based therapy in clinical practice with a Korean cohort. Whether rifaximin could provide additional benefit for the eradication of H. pylori was assessed. The result showed that the levofloxacin and rifaximin based quadruple regimen was not inferior to the standard first-line therapy, whereas the levofloxacin based regimen showed limited efficacy. It is surprising that the levofloxacin-based triple regimen was inferior to the standard first-line therapy. In the past few years, a triple therapy including a levofloxacin-amoxycillin combination has been proposed for either first-line or rescue therapy regimen to cure H. pylori infection (28). This finding could depend on the higher prevalence of primary levofloxacin resistance. Unfortunately, the prevalence of primary levofloxacin resistance rate is increasing up to 21.5% in Korea (29). Such an increase in the bacterial resistance against levofloxacin probably comes from treatment of pulmonary or urinary infections with quinolones.
However, several methodological weaknesses may limit the validity of these findings. First, a major limitation of this study was the single center collection of data, raising concern about the ability to generalize the results. Second, we did not measure antibiotic resistance. Third, the CYP2C19 genotypes were not considered as a potential influence on the outcome of eradication therapy. The principal enzyme involved in the metabolism of the PPI's is CYP2C19, and there are inter-individual differences in the activity of this enzyme (30). A large, multicenter-based comparative study is needed to confirm the findings.
Antibiotic-associated gastrointestinal adverse effects such as diarrhea, nausea, vomiting, bloating, and abdominal pain may cause patients to interrupt the prescribed regimen. This can lead to bacterial resistance. The frequency and severity of individual symptoms during eradication therapy were analyzed in this study. The frequency of diarrhea, taste disturbance, and abdominal pain were similar in all three groups. Moderate to severe adverse effects were more common with the standard therapy. Of note, was the significant safety profile of rifaximin. When diarrhea occurs with eradication therapy it is usually transient and self limiting; however, occasionally, severe effects occur. Several cases of Clostridium difficile infection have been reported. The results of this study showed that diarrhea was the most common complaint during eradication therapy and the most important factor associated with drug compliance. The regimen with rifaximin had a significantly lower frequency of moderate to severe diarrhea. However, it had limited efficacy compared to the standard first-line therapy. In the near future, high-dose regimens (400 mg, tid) or longer duration (e.g. 10 or 14 days) will be tested with rifaximin with the hope of achieving better eradication rates.
In summary, the levofloxacin and rifaximin based regimen comes up to the standard triple therapy but has limited efficacy in a Korean cohort. When rifaximin is added to amoxicillin or levofloxacin at a sub-inhibitory concentration, synergistic effect on H. pylori eradication therapy is not observed. However, the rifaximin containing regimen has an excellent safety profile and might have improved efficacy at a full dose of it or in combination with other antibiotics.
Figures and Tables
 | Fig. 1Study flow diagram. OAC, omeprazole amoxicillin clarithromycin; OAL, omeprazole amoxicillin levofloxacin; OAL-R, omeprazole amoxicillin levofloxacin rifaximin. |
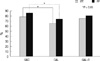 | Fig. 2Eradication rate. The eradication rate achieved with the OAL regimen was significantly lower than with the standard OAC regimen in the ITT and PP analysis (P < 0.05). OAC, omeprazole amoxicillin clarithromycin; OAL, omeprazole amoxicillin levofloxacin; OAL-R, omeprazole amoxicillin levofloxacin rifaximin; ITT, intention-to-treat; PP, per-protocol. |
 | Fig. 3Frequency of adverse effects in eradication therapy. (A) No significant differences in frequency of adverse effects were found among the groups. (B) The frequency of moderate to severe adverse effects was significantly higher with the OAC regimen. Diarrhea was the most common complaint. Moderate to severe diarrhea was significantly low with the OAL-R regimen. OAC, omeprazole amoxicillin clarithromycin; OAL, omeprazole amoxicillin levofloxacin; OAL-R, omeprazole amoxicillin levofloxacin rifaximin. |
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. In-Sik Chung for his advice. All of the authors have no conflict of interest with this study.
AUTHOR SUMMARY
Efficacy of Levofloxacin and Rifaximin based Quadruple Therapy in Helicobacter pylori Associated Gastroduodenal Disease: A Double-Blind, Randomized Controlled Trial
Kang Hyun Choi, Woo Chul Chung, Kang-Moon Lee, Chang Nyol Paik, Eun Jung Kim, Bong Koo Kang, Ju Hyun Oak and Sung Hoon Jung
The standard triple therapy for H. pylori infection, which includes proton pump inhibitor (PPI), amoxicillin and clarithromycin fails in up to 20% of patients or even higher in some clinical setting. We aim to determine the efficacy of levofloxacin in Korea. Additionally, the combination of rifaximin is studied for its synergistic effects for H. pylori eradication. The levofloxacin and rifaximin based regimens come up to the standard triple therapy but had limited efficacy in a Korean cohort. In the process of therapy, diarrhea was the most common complaint. The rifaximin containing regimen had a significantly lower frequency of moderate to severe diarrhea. In the near future, high-dose regimens (400 mg, tid) or longer duration (e.g. 10 or 14 days) will be tested with rifaximin with the hope of achieving better eradication rates.
References
1. Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ Jr, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, coltrolled study. Ann Intern Med. 1992. 116:705–708.
2. Lind T, Mégraud F, Unge P, Bayerdörffer E, O'morain C, Spiller R, Veldhuyzen Van Zanten S, Bardhan KD, Hellblom M, Wrangstadh M, Zeijlon L, Cederberg C. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology. 1999. 116:248–253.
3. Bochenek WJ, Peters S, Fraga PD, Wang W, Mack ME, Osato MS, El-Zimaity HM, Davis KD, Graham DY. Helicobacter pylori Pantoprazole Eradication (HELPPE) Study Group. Eradication of Helicobacter pylori by 7-day triple-therapy regimens combining pantoprazole with clarithromycin, metronidazole, or amoxicillin in patients with peptic ulcer disease: results of two double-blind, randomized studies. Helicobacter. 2003. 8:626–642.
4. Perri F, Villani MR, Festa V, Quitadamo M, Andriulli A. Predictors of failure of Helicobacter pylori eradication with the standard 'Maastricht triple therapy'. Aliment Pharmacol Ther. 2001. 15:1023–1029.
5. Queiroz DM, Dani R, Silva LD, Santos A, Moreira LS, Rocha GA, Corrêa PR, Reis LF, Nogueira AM, Alvares Cabral MM, Esteves AM, Tanure J. Factors associated with treatment failure of Helicobacter pylori infection in a developing country. J Clin Gastroenterol. 2002. 35:315–320.
6. Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy-the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999. 13:1047–1055.
7. van der Wouden EJ, Thijs JC, van Zwet AA, Sluiter WJ, Kleibeuker JH. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999. 94:1751–1759.
8. Huang DB, DuPont HL. Rifaximin - a novel antimicrobial for enteric infections. J Infect. 2005. 50:97–106.
9. Quesada M, Sanfeliu I, Junquera F, Segura F, Calvet X. Evaluation of Helicobacter pylori susceptibility to rifaximin. Gastroenterol Hepatol. 2004. 27:393–396.
10. Gasbarrini A, Lauritano EC, Nista EC, Candelli M, Gabrielli M, Santoro M, Zocco MA, Cazzato A, Finizio R, Ojetti V, Cammarota G, Gasbarrini G. Rifaximin-based regimens for eradication of Helicobacter pylori: a pilot study. Dig Dis. 2006. 24:195–200.
11. Tanaka M, Isogai E, Isogai H, Hayashi S, Hirose K, Kimura K, Sugiyama T, Sato K. Synergic effect of quinolone antibacterial agents and proton pump inhibitors on Helicobacter pylori. J Antimicrob Chemother. 2002. 49:1039–1040.
12. Assem M, El Azab G, Rasheed MA, Abdelfatah M, Shastery M. Efficacy and safety of levofloxacin, clarithromycin and esomeprazol as first line triple therapy for Helicobacter pylori eradication in Middle East. Prospective, randomized, blind, comparative, multicenter study. Eur J Intern Med. 2010. 21:310–314.
13. Lee JH, Hong SP, Kwon CI, Phyun LH, Lee BS, Song HU, Ko KH, Hwang SG, Park PW, Rim KS, Kim S. The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2006. 48:19–24.
14. Malfertheiner P, Mégraud F, O'Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ, Pajares J, Quina M, Rauws E. The European Helicobacter Pylori Study Group (EHPSG). Current European concepts in the management of Helicobacter pylori infection - the Maastricht Consensus Report. Eur J Gastroenterol Hepatol. 1997. 9:1–2.
15. Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. European Helicobacter Pylori Study Group (EHPSG). Current concepts in the management of Helicobacter pylori infection-the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002. 16:167–180.
16. Houben MH, van de Beek D, Hensen EF, Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy-the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999. 13:1047–1055.
17. Sasaki M, Ogasawara N, Utsumi K, Kawamura N, Kamiya T, Kataoka H, Tanida S, Mizoshita T, Kasugai K, Joh T. Changes in 12-year first-line eradication rate of Helicobacter pylori based on triple therapy with proton pump iInhibitor, amoxicillin and clarithromycin. J Clin Biochem Nutr. 2010. 47:53–58.
18. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010. 59:1143–1153.
19. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007. 56:772–781.
20. Xia HX, Buckley M, Keane CT, O'Morain CA. Clarithromycin resistance in Helicobacter pylori: prevalence in untreated dyspeptic patients and stability in vitro. J Antimicrob Chemother. 1996. 37:473–481.
21. Mégraud F. Strategies to treat patients with antibiotic resistant Helicobacter pylori. Int J Antimicrob Agents. 2000. 16:507–509.
22. Alarcón T, Vega AE, Domingo D, Martínez MJ, López-Brea M. Clarithromycin resistance among Helicobacter pylori strains isolated from children: Prevalence and study of mechanism of resistance by PCR-restriction fragment length polymorphism analysis. J Clin Microbiol. 2003. 41:486–499.
23. Mégraud F. H. pylori antimicrobial resistance: prevalence, importance, and advances in testing. Gut. 2004. 53:1374–1384.
24. Eun CS, Han DS, Park JY, Jeon YC, Hahm JS, Kim KS, Kang JO. Changing pattern of antimicrobial resistance of Helicobacter pylori in Korean patients with peptic ulcer diseases. J Gastroenterol. 2003. 38:436–441.
25. Bang SY, Han DS, Eun CS, Kim JE, Ahn SB, Sohn JH, Jeon YC, Kang JO. Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease. Korean J Gastroenterol. 2007. 50:356–362.
26. Sung H, Chung HJ, Kim MN, Lee GH. Clinical usefulness of antimicrobial susceptibility test for Helicobacter pylori. Korean J Lab Med. 2006. 26:179–184.
27. Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am J Gastroenterol. 2009. 104:21–25.
28. Wong WM, Gu Q, Lam SK, Fung FM, Lai KC, Hu WH, Yee YK, Chan CK, Xia HH, Yuen MF, Wong BC. Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy versus quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003. 17:553–560.
29. Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006. 28:6–13.
30. Lee JH, Jung HY, Choi KD, Song HJ, Lee GH, Kim JH. The influence of CYP2C19 polymorphism on eradication of Helicobacter pylori: a prospective randomized study of lansoprazole and rabeprazole. Gut Liver. 2010. 4:201–206.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download