Abstract
Scutellarin is a flavonoid extracted from a traditional Chinese herb, Erigeron breviscapus. The present study investigated the effect of scutellarin on MUC5AC mucin production and the possible mechanism. Human bronchial epithelial 16 (HBE16) cells were pretreated with scutellarin for 60 min, and then exposed to human neutrophil elastase (HNE) or interleukin (IL)-13 for 12 hr. RT-PCR and ELISA were performed to measure the amount of MUC5AC mucin production. The results showed that scutellarin inhibited MUC5AC expression both in mRNA and protein level induced by HNE in a concentration-dependent manner. However, scutellarin failed to inhibit MUC5AC mucin production induced by IL-13. To investigate the intracellular mechanisms associated with the effect of scutellarin on MUC5AC mucin production, western blotting was carried out to examine the phosphorylation of protein kinase C (PKC), signal transducer and activator of transcription 6 (STAT6) and extracellular signal-regulated kinase 1/2 (ERK1/2). The phosphorylation of PKC and ERK1/2 was attenuated after treatment with scutellarin, whereas STAT6 was not significantly affected. Therefore, it is suggested that scutellarin down-regulates MUC5AC mucin production on HBE16 cells via ERK-dependent and PKC-dependent pathways.
The excess mucus production is an important feature of chronic inflammatory diseases, including chronic bronchitis, bronchiectasis and asthma. Mucus overproduction contributes to morbidity and mortality in many patients, particularly in those with more severe disease. During acute exacerbation of chronic obstructive pulmonary disease (COPD), human neutrophil elastase (HNE), reactive oxygen species (ROS) and many cytokines may injure the airway and activate mucin gene regulation (1). Excessive mucus in the airways is linked to an increase in the frequency and duration of infection and a decline in lung function. More than 20 human mucin genes have been deposited in the GenBank. Among all these mucin in mammals, mucin (MUC) 5AC and MUC5B are produced significantly in intrapulmonary airways. Up-regulated MUC5AC expression is the central event in goblet cell metaplasia (2). HNE has been shown to be a potent secretagogue for goblet cells in vitro and in vivo. HNE increases the transcriptional activity of MUC5AC promoter and MUC5AC mRNA expression through mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathways. HNE is also known to elevate the MUC5AC mRNA levels by enhancing mRNA stability or inducing ROS (3, 4). Several cytokines including tumor necrosis factor, interleukin (IL)-1β, and IL-13 upregulate MUC5AC expression. IL-13, a central mediator of airway remodeling in asthma, increases MUC5AC expression by the phosphorylation of signal transducer and activator of transcription 6 (STAT6) and the suppression of the transcription factor, Forkhead boxA2 (FOXA2) (5).
Mucoregulatory medications decrease mucin secretion or impede the formation of the DNA/F-actin secondary polymer network. The most thoroughly studied mucoregulatory medications are the low-dose macrolide antibiotics. The mechanism of that medication appears to be through the modulation of the MAPK/ERK cascade (6). However, rapid spread of bacterial resistance has been observed after overdose medication or the inaccurate treatment. Moreover, the gastrointestinal adverse events of macrolide antibiotics occurred frequently, including gastroesophageal reflux and functional dyspepsia. All these problems have impeded the application of the macrolide antibiotic as an effective treatment for mucus hypersecretion. No current safety and effective mucoregulatory medications is developed in clinical management of patients with COPD or asthma. In these clinical situations, it is noteworthy to find novel therapeutic approaches to decrease mucus hypersecretion.
It was reported that several natural compounds such as genistein and curcumin could affect mucus production in the airway. Ram and his colleagues reported that curcumin attenuated allergen induced airway hyperresponsiveness in sensitized guinea pigs (7). Also, Heo and his colleagues (8) reported that genistein and curcumin suppressed epidermal growth factor induced MUC5AC mucin production and gene expression from human airway epithelial cells. Thus, natural compounds might provide possible approaches to prevent mucus hypersecretion.
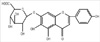
Scutellarin (scu) (Fig. 1), a known flavone 7-O-β-D-glucuronide with a molecular weight of 462.21, is considered as the primary active ingredient of breviscapine. It has been extensively used to treat ischemic cerebrovascular and cardiovascular diseases (9, 10). Scutellarin has been observed to possess comprehensive pharmacological functions, including dilating vessels, inhibiting platelet aggregation and reducing intracellular free Ca2+ levels (11). Nowadays, various preparations of scutellarin are still widely used clinically to treat cerebrovascular and cardiovascular diseases such as hypertension, angina pectoris, coronary heart disease, cerebral ischemia and vascular dementia. Scutellarin also possesses strong ability to inhibit protein kinase C (PKC) (12, 13), which is involved in the regulation of MUC5AC expression in vivo and in vitro (14, 15). Therefore, we hypothesize that scutellarin may decrease MUC5AC mucin production in airway epithelial cells.
In this study, the immortalized human bronchial epithelial cell line (HBE-16) were pretreated with scutellarin for 60 min and then exposed to HNE or IL-13 for 12 hr. MUC5AC mucin product was assessed by RT-PCR and ELISA. We investigated intracellular mechanisms and signaling pathways associated with the effects of scutellarin on MUC5AC mucin production. The mechanism appears to involve the inhibition of PKC and ERK1/2.
All the antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) unless otherwise specified. All the chemicals and reagents were from Sigma (St. Louis, MO, USA) unless otherwise indicated. Calphostin C (a specific PKC inhibitor) and PD98059 (a specific ERK inhibitor) were purchased from Calbiochem (San Diego, CA, USA). A771726 were purchased from Enzo Life Sciences. The HNE inhibitor elastatinal was obtained from Calbiochem (La Jolla, CA, USA). Scutellarin (purity > 95%) was purchased from Wangzilong Pharmaceutical Co. LTD (Yuxi, Yunnan, China). Scutellarin was dissolved in DMSO and diluted with saline before use. The final concentration of DMSO was less than 0.1%.
HBE-16 cells were maintained in RPMI-1640 medium (Gibco-Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal calf serum (Gibco) and 1% weight per volume penicillin/streptomycin (Gibco). Cell cultures were maintained in a humidified atmosphere of 5% CO2 in air at 37℃. The medium was changed till cells were 90% confluent.
The cells were seeded in 6-well plate at 5 × 105 cells/well. After the cells reached 90% confluence, they were serum starved for 24 hr to maintain a low basal level of MUC5AC mucin production. To determine the effects of HNE (Elastin Products, Owensville, MO, USA) or IL-13 (Peprotech, Hill, NJ, USA), cells were exposed to 0.01-1 µM/L HNE or 1-25 ng/mL recombinant human IL-13 for 12 hr (15, 16).
To investigate the concentration effect of scutellarin on the cell, the cells were incubated in RPMI-1640 medium containing scutellarin with the concentration from 10 µM/L to 100 µM/L for 60 min (17) and then exposed to 0.1 µM/L HNE or 10 ng/mL recombinant human IL-13 for 12 hr. In the positive control group, Elastatinal (100 µM/L) was added to the medium before exposure to HNE and A771726 (10 µM/L) was used as an inhibitor against IL-13. In the negative control group, the cells were incubated with medium alone. At the end of stimulation, cells were collected for further evaluations.
Total RNA was extracted from HBE16 cells after stimulation with HNE or IL-13 for 12 hr using ISOGEN (Gibco-Invitrogen). Oligonucleotide primers for PCR were designed according to the published sequence for human MUC5AC (sense: TCA ACG GAG ACT GCG AGT ACA C; antisense: TCT TGA TGG CCT TGG AGC A). The PCR conditions were as follows: a pre-denaturing at 94℃ for 10 min, followed by 33 cycles of denaturation at 94℃ for 30 sec, annealing at 59℃ for 30 sec and extension at 72℃ for 30 sec. PCR products were separated by electrophoresis through 1% agarose gel containing ethidium bromide, and the signal intensity was analyzed by BIO-RAD Image. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) controls were used to standardize the quantification of RNA samples.
The MUC5AC protein was measured by enzyme-linked immunosorbent assay (ELISA). The cells were lysed in a standard RIPA buffer, containing protease inhibitors. All the samples were incubated at 42℃ in a 96-well plate for 1 hr and 4℃ overnight. The plates were washed three times with PBS and blocked with 2% BSA for 1 hr at room temperature. After washing the plates for three times with PBS, the wells were incubated in PBS with mouse monoclonal antibody against MUC5AC (NeoMarkers, Fremont, CA, USA) for 1 hr, which was diluted with PBS containing 0.05% Tween 20. Then, the wells were washed three times with PBS, and 100 µM of horseradish peroxidase-goat anti-mouse IgG conjugate was dispensed into each well for 1 hr. The plates were washed three times with PBS and the color reaction was developed with 3, 3', 5, 5'-tetramethylbenzidine peroxide solution and stopped with 50 mM/L H2SO4. The absorbance was read at 405 nm wavelengths using a synergy HT multi-detection microplate reader.
The phosphorylation PKC, ERK1/2 and STAT6 were examined by western analysis. Cells were collected at the end of exposure. Protein concentrations were determined by Bio-Rad assays. Cells lysate was fractionated on SDS-PAGE, and proteins were then transferred to a nitrocellulose membrane (Millipore, Billerica, MA, USA). Membranes were blocked with 5% FBS in Tris-buffered saline containing 0.1% Tween 20 at room temperature for 1 hr and then incubated overnight at 4℃ with the mouse antibodies against p-PKC, p-ERK1/2 and p-STAT6. GAPDH protein was used to normalize the total tissue lysate on the same nitrocellulose membrane. After three washed, membranes were incubated for 1 hr at room temperature with horseradish peroxidase conjugate goat anti-mouse IgG antibody. The three washes were repeated, and then protein was visualized with enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
The results were expressed as mean ± SEM. Differences were examined for statistical significance using one-way analysis of variance. The post hoc test was Fisher's protected least significant difference (PLSD) test. A P value < 0.05 denoted the presence of a statistically significant difference.
To confirm whether HNE or IL-13 can induce MUC5AC mucin production on HBE-16 cells, we first evaluated the MUC5AC protein expression after addition of HNE or IL-13 to the cells at various concentrations. We found that the stimulation with HNE led to a concentration-dependent increase in MUC5AC production compared with the control group (Fig. 2). The expression of MUC5AC protein also increased as a result of IL-13 stimulation in a concentration dependent manner (Fig. 3).
We next evaluated the effects of scutellarin on MUC5AC expression induced by HNE. RT-PCR and ELISA were used to examine the expression of MUC5AC production regulated by scutellarin. The cells were pretreated with scutellarin for 60 min before the addition of 0.1 µM/L NHE. In the HNE control group, the cells were stimulated with HNE after treatment with medium only. As shown in Fig. 4, scutellarin significantly reduced the enhanced expression of MUC5AC induced by HNE at both the mRNA and protein levels. In the positive control group, elastatinal greatly reduced the MUC5AC synthesis induced by HNE (data not shown).
The mRNA and protein expression of MUC5AC decreased as a result of scutellarin inhibition in a concentration dependent manner. Half-maximal inhibition effect was elicited after incubation with scutellarin at the concentration of 50 µM/L (Fig. 4B). Therefore, concentration of 50 µM/L was chosen for the following experiments.
To determine whether scutellarin could inhibit mucus production induced by IL-13, MUC5AC synthesis was assayed using RT-PCR and ELISA. The cells were pretreated with scutellarin for 60 min before the addition of 10 ng/mL IL-13. In the IL-13 control group, the cells were stimulated with IL-13 after treatment with medium only. As shown in Fig. 5, scutellarin did not reduce the MUC5AC synthesis induced by IL-13 at both the mRNA and protein levels.
We investigated the possible involvement of PKC in the protective activity of scutellarin against mucus secretion. The cells were preincubated with 0.1 µM/L calphostin C or 50 µM/L scutellarin for 60 min before exposed to 0.1 µM/L HNE for 1 hr. As shown in Fig. 6, PKC activity induced by HNE was attenuated when the cells were pretreated with calphostin C. Compared with the HNE control group, a significant decrease in the phosphorylation of PKC was also observed when the cells were pretreated with scutellarin.
To study the involvement of ERK1/2 in the protective activity of scutellarin against MUC5AC mucin production induced by HNE, the cells were stimulated with HNE for 1 hr after treatment with 50 µM/L scutellarin or 50 µM/L PD98059 for 60 min. As shown in Fig. 7, Pretreatment with PD98059 resulted in significant decreases in ERK1/2 phosporylation when exposed to HNE. Compared with the HNE control group, the phosphorylation of ERK1/2 also significantly decreased after treatment with scutellarin.
For STAT6 group, the cells were pretreated with 50 µM/L scutellarin or 10 µM/L A771726 for 60 min before exposed to 10 ng/L IL-13 for 1 hr. As shown in Fig. 8, A771726 significantly decreases STAT6 phosphorylation responding to IL-13. In contrast, pretreatment with scutellarin failed to interrupt STAT6 phosphorylation when exposed to IL-13. These data suggest that MAPK/ERK signal pathway, not STAT6 pathway, may play an important role in the inhibition of scutellarin on MUC5AC mucin production induced by HNE.
Excessive mucus secretion is problematic in patients with chronic airway diseases due to airway obstruction and impairment of gas exchange. In this study, we carried out experiments to examine whether treatment with scutellarin might attenuate mucus production induced by HNE or IL-13. Our study demonstrated that: 1) stimulation with HNE or IL-13 increased the expression of MUC5AC; 2) scutellarin significantly attenuated the MUC5AC expression at both the mRNA and protein levels induced by HNE. However, scutellarin failed to inhibit the MUC5AC expression induced by IL-13; 3) PKC played an important role in mucus secretion and was involved in the inhibition of scutellarin against MUC5AC mucin production induced by HNE; 4) MAPK/ERK, but not STAT6 pathway, was implicated in the effects of scutellarin on mucus production.
Our study demonstrated that HNE and IL-13 induced MUC5AC expression on HBE-16 cells. HNE is the most widely studied with regard to enhanced mucus secretion. Purified HNE has been shown to provoke secretion of mucin by isolated airway epithelial cells and glands from several species (3, 4, 15). Mucin protein is also upregulated by IL-13, which plays a central role in the pathogenesis of chronic inflammatory diseases. Previous studies have indicated that IL-13 could induce mucus secretion in mouse airways via the expression of both the IL-13 receptor and the IL-13 signaling molecule signal transducer (18). Up-regulated signaling through STAT6 pathway plays an important role in mucus production induced by IL-13 (5, 16).
After the confirmation of the effect of HNE or IL-13 on mucus secretion on HBE16 cells, we investigate the effect of scutellarin on MUC5AC mucin production induced by HNE or IL-13. Compared with calphostin C, scutellarin showed an equipotent effect in the inhibition of MUC5AC mucin production induced by HNE. However pretreatment with scutellarin did not reduce IL-13 induced MUC5AC production. To investigate the reason for this discrepancy with the effect of scutellarin against MUC5AC production induced by HNE or IL-13, we examined the effects of scutellarin on MAPK/ERK signaling transduction and STAT6 signal transduction. Scutellarin attenuated the phosphorylation of ERK1/2, which was significantly phosphorylated after the stimulation of HNE. Pretreatment with scutellarin failed to attenuate the phosphorylation of STAT6 induced by IL-13. In contrast, A771726, a specific STAT6 inhibitor, greatly decreased the phosphorylation of STAT6 and the expression of MUC5AC induced by IL-13. Taken together, we identified that scutellarin down-regulated MUC5AC production induced by HNE through MAPK/ERK signal transduction. Scutellarin could not affect mucus production induced by IL-13 and STAT6 pathway was not involved in the mechanism of scutellarin on MUC5AC production.
Among a variety of signal transduction molecules, MAPK has been shown to play an important role in mucus secretion (19). Kuwahara demonstrated that a PKC → ERK1/2 → Sp1 pathway was responsible for HNE-activated mucus secretion (20). Our study showed that such pathway might be related to the protective effect of scutellarin against MUC5AC production. Pan et al. (11) reported that suppression of ERK1/2 activation by scutellarin represented one of the possible mechanisms accounting for its beneficial effects on impaired cardiac function after myocardial infarction. Our results are consistent with the conclusion and clearly reveal that scutellarin blocked ERK pathway activated by HNE.
PKC was known to be involved in secretion of airway mucin responding to various stimuli (14). It has been reported that scutellarin has the strong inhibitory effects on PKC activation, which contributes to its protective role in cerebral ischemia and hepatic injury during brain-death (12, 13). To confirm whether PKC was involved in the inhibition of scutellarin against mucus production on HBE16, phosphorylation of PKC was determined by western blotting. Our data clearly revealed that scutellarin blocked PKC activation induced by HNE. Inhibition of PKC attenuated HNE-mediated mucus secretion. Therefore, the effect of scutellarin on MUC5AC mucin production can be part ascribed to its specifically blocking PKC activation.
The mechanism whereby scutellarin inhibit PKC is uncertain. Many studies have shown that scutellarin is a Ca2+ channel-blocking agent with the ability to inhibit extracellular calcium influx (11, 21). Pan et al. (22) reported scutellarin exerted its anti-hypertrophic effects via suppressing the Ca2+-mediated calcineurin. Hong et al. (23) reported that scutellarin protected against hydrogen peroxide-induced cytotoxicity in PC12 cells via reducing intracellular accumulation of Ca2+. Considering calcium as a robust activator of PKC, the inhibition of scutellarin on PKC phosphorylation might be associated with its ability to block Ca2+ channel. Interestingly, correlation between calcium channels and mucus expression in airways has suggested a causal relationship. Transfection of hCLCA1 into human mucoepidermoid cells resulted in up-regulation of the MUC5AC gene (24). Macrolide antibiotics, the most thoroughly studied mucoregulatory medications, may inhibit mucus secretion via reducing intracellular accumulation of Ca2+ (25). Therefore, the molecular mechanism of scutellarin against mucus secretion might be associated with its ability to block calcium channel. Further studies are required to investigate the specific mechanism.
In conclusion, the present study has demonstrated that scutellarin inhibited MUC5AC mucin production via inhibiting PKC activation and diminishing the phosphorylation of ERK1/2. Our results provide experimental evidence for the use of scutellarin in the clinic as effective means for treatment of mucus hypersecretion. Indeed, scutellarin shows promising effect on respiratory disease and possesses wide-ranging positive effects on the control of mucus secretion. Importantly, the curative dosage of scutellarin in clinic is safe, without liver or kidney toxicity. It has been used in cardiovascular disease and cerebral infarction with satisfactory tolerance and safety (26). In addition to this, it is supposed that scutellarin is a potential effective and safety candidate in the regulation of mucus secretion.
Figures and Tables
 | Fig. 2Up-regulation of MUC5AC protein induced by human neutrophil elastase (HNE) on HBE16 cells. Cells were collected after exposed to HNE at different concentrations. The amount of MUC5AC was measured by ELISA. Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative control group. |
 | Fig. 3Up-regulation of MUC5AC induced by IL-13 on HBE16 cells. Cells were collected after exposed to IL-13 at different concentrations. The amount of MUC5AC was measured by ELISA. Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative control group. |
 | Fig. 4Effects of scutellarin (scu) on MUC5AC mRNA (A) and protein (B) expression after exposed to HNE. The cells were pretreated with scu or medium only for 60 min before the addition of 0.1 µM/L HNE. Total RNAs were reverse transcribed and used for PCR amplication (A). The amount of MUC5AC protein was measured by ELISA (B). Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative group; †P < 0.05 compared with the HNE control group. |
 | Fig. 5Effects of scutellarin (scu) on MUC5AC mRNA (A) and protein (B) expression after exposed to IL-13. The cells were pretreated with scu or medium only for 60 min before the addition of 10 ng/mL IL-13. Total RNAs were reverse transcribed and used for PCR amplication (A). The amount of MUC5AC protein was measured by ELISA (B). Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative control group; ‡P > 0.05 compared with the IL-13 control group. |
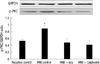 | Fig. 6Effects of scutellarin (scu) on the phosphorylation of PKC induced by HNE. The cells were pretreated with scu, Calphostin C or medium only for 60 min before the addition of 0.1 µM/L HNE. The phosphorylation of PKC was measured by western. Data represent means ± SEM of four experiments. *P < 0.05 compared with the control group; †P < 0.05 compared with the HNE control group. |
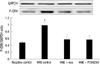 | Fig. 7Effects of scutellarin (scu) on the phosphorylation of ERK1/2 induced by HNE. The cells were pretreated with scu, PD98059 or medium only for 60 min before the addition of 0.1 µM/L HNE. The phosphorylation of ERK1/2 was measured by western. Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative group; †P < 0.05 compared with the HNE control group. |
 | Fig. 8Effects of scutellarin (scu) on the phosphorylation of STAT6 induced by IL-13. The cells were pretreated with scu, A771726 or medium only for 60 min before the addition of 10 ng/mL IL-13. The phosphorylation of STAT6 was measured by western. Data represent means ± SEM of four experiments. *P < 0.05 compared with the negative group; †P < 0.05 compared with the IL-13 control group; ‡P > 0.05 compared with the IL-13 control group. |
AUTHOR SUMMARY
Effects of Scutellarin on MUC5AC Mucin Production Induced by Human Neutrophil Elastase or Interleukin 13 on Airway Epithelial Cells
De-Peng Jiang, Juliy M. Perelman, Victor P. Kolosov and Xiang-Dong Zhou
Mucus hypersecretion is clinically critical problem of various airway diseases. The present study demonstrates that scutellarin inhibited MUC5AC mucin production via inhibiting PKC activation and diminishing the phosphorylation of ERK1/2. Our results provide experimental evidence for a plausibility of clinical trial of scutellarin treatment for the mucus hypersecretion. Our results suggest that the clinically applicable dosage of scutellarin could be safe, without liver or kidney toxicity. As a whole, we suppose that scutellarin is a potentially effective and safety candidate in the regulation of mucus secretion.
References
1. Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, Papi A. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004. 45:477–484.
2. Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, Adachi R, Blackburn MR, Dickey BF, Evans CM. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements. Am J Respir Cell Mol Biol. 2007. 37:273–290.
3. Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1293–L1302.
4. Shao MX, Nadel JA. Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha- converting enzyme. J Immunol. 2005. 175:4009–4016.
5. Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K, Ise H, Yamaya M. Modulation of mucus production by interleukin-13 receptor alpha 2 in the human airway epithelium. Clin Exp Allergy. 2008. 38:122–134.
6. Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther. 2008. 117:393–405.
7. Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003. 26:1021–1024.
8. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009. 23:1458–1461.
9. Gao ZX, Huang DY, Li HX, Zhang LN, Lv YH, Cui HD, Zheng JH. Scutellarin promotes in vitro angiogenesis in human umbilical vein endotheli al cells. Biochem Biophys Res Commun. 2010. 400:151–156.
10. Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharmacol. 2007. 50:327–332.
11. Pan Z, Zhao W, Zhang X, Wang B, Wang J, Sun X, Liu X, Feng S, Yang B, Lu Y. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of infarct rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. Br J Pharmacol. 2011. 162:688–700.
12. Xu W, Zha RP, Wang WY, Wang YP. Effects of scutellarin on PKCgamma in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharmacol Sin. 2007. 28:1573–1579.
13. Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009. 30:1081–1091.
14. Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol Lung Cell Mol Physiol. 2003. 285:L149–L160.
15. Park JA, He F, Martin LD, Li Y, Chorley BN, Adler KB. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase C{delta}-mediated mechanism. Am J Pathol. 2005. 167:651–661.
16. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007. 36:244–253.
17. Yan L, Huang H, Tang QZ, Zhu LH, Wang L, Liu C, Bian ZY, Li H. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem. 2010. 109:1158–1171.
18. Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, Cohn L. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am J Respir Cell Mol Biol. 2002. 27:593–602.
19. Rhee CK, Kang CM, You MB, Yoon HK, Kim YK, Kim KH, Moon HS, Park SH, Song JS. Effect of fudosteine on mucin production. Eur Respir J. 2008. 32:1195–1202.
20. Kuwahara I, Lillehoj EP, Lu W, Singh IS, Isohama Y, Miyata T, Kim KC. Neutrophil elastase induces IL-8 gene transcription and protein release through p38/NF-{kappa}B activation via EGFR transactivation in a lung epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L407–L416.
21. Zhu BH, Ma L, Pan XD, Huang YL, Liu J. Scutellarin induced Ca(2+) release and blocked KCl-induced Ca(2+) influx in smooth muscle cells isolated from rat thoracic artery. J Asian Nat Prod Res. 2008. 10:583–589.
22. Pan ZW, Zhang Y, Mei DH, Zhang R, Wang JH, Zhang XY, Xu CQ, Lu YJ, Yang BF. Scutellarin exerts its anti-hypertrophic effects via suppressing the Ca2+-mediated calcineurin and CaMKII signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2010. 381:137–145.
23. Hong H, Liu GQ. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004. 74:2959–2973.
24. Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci USA. 2001. 98:5175–5180.
25. Lu S, Liu H, Farley JM Sr. Macrolide antibiotics inhibit mucus secretion and calcium entry in swine airway submucosal mucous gland cells. J Pharmacol Exp Ther. 2011. 336:178–187.
26. Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009. 30:1081–1091.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download