Abstract
Aspirin is a kind of anti-inflammatory drug and may be used to reverse hyperglycemia, hyperinsulinemia, and dyslipidemia by improving insulin resistance. We hypothesized that aspirin improves insulin resistance in type 2 diabetes by inhibiting hepatic nuclear factor kappa-β (NF-κB) activation and serum tumor necrosis factor-α (TNF-α). Adult male Wistar rats were randomly divided into four groups: control, untreated diabetic, diabetic treated with metformin (100 mg/kg/day), and diabetic treated with aspirin (120 mg/kg/day). Diabetes was induced by high-fat feeding and a low dose of streptozotocin (30 mg/kg). After treatment, plasma glucose, insulin, lipids, free fatty acids (FFAs) concentrations and serum TNF-α were determined. The expression of NF-κB in hepatocytes was analyzed by immunohistochemistry and western blot. The results showed administration of aspirin caused no significant lowering in fasting glucose level but significant reduction of hepatic NF-κB expression and serum TNF-α level with improved insulin resistance compared to the diabetic group. The relevant analysis showed positive correlation between the expression of homeostasis model assessment-insulin resistance (HOMA-IR) and NF-κB (r = 0.799, P < 0.01); HOMA-IR and serum TNF-α (r = 0.790, P < 0.01). It is concluded that aspirin improves insulin resistance by inhibiting hepatic NF-κB activation and TNF-α level in streptozotocin-induced type 2 diabetic rats.
A lot of evidence suggests that, insulin resistance, defined as a state of reduced insulin action in peripheral tissues, such as the skeletal muscle, adipose tissue, and liver, plays a critical role in the development and onset of type 2 diabetes (1, 2). In recent years, more evidence has emerged that proinflammatory cytokines can cause sustained development of insulin resistance (3) and anti-inflammatory medications may reverse the process of it (4, 5). A number of epidemiological studies and animal research have shown a correlation between insulin resistance and inflammatory markers such as tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) and interleukin-6 (IL-6) (6, 7), suggesting that inflammation may be directly involved in the pathogenesis of insulin resistance. TNF-α, produced by adipocytes and macrophages, has been implicated to play an important role in the cascade of inflammation, systemic insulin resistance, reduced β-cell secretion of insulin, and thus type 2 diabetes (8). Nuclear factor kappa-β (NF-κβ) is a nuclear transcriptional activator that also plays a central role in the inflammation. Recently there were two studies supporting the important function of NF-κB in insulin resistance. Elevated expression of the IKK-β (NF-κB kinase) in hepatic cells caused profound hepatic insulin resistance and more moderate systemic insulin resistance in mice (9). Mice lacking IKK-β in hepatocytes retained global insulin sensitivity and were protected from insulin resistance in response to high-fat diet, obesity, or aging (10). Substantial evidence indicates that aspirin, a kind of non-steroidal anti-inflammatory drug (NSAIDs), has antipyretic, analgesic and anti-inflammatory effects.
Recent studies indicated that aspirin might be an insulin-sensitizing agent and they may be used to reverse hyperglycemia, hyperinsulinemia, and dyslipidemia by sensitizing insulin signaling and improving insulin resistance (4). However, the cellular and molecular mechanisms of aspirin in improving insulin resistance in type 2 diabetes have not been well elucidated. We therefore hypothesized that aspirin improves insulin resistance in type 2 diabetes by inhibiting NF-κB activation and TNF-α levels. To test this hypothesis, we examined the effect of aspirin on hepatic NF-κB activation and its downstream target gene TNF-α in rat model of type 2 diabetes induced by high-carbohydrate-fat diet and streptozotocin (STZ).
STZ was purchased from Sigma (St. Louis, MO, USA); insulin was purchased from Eli Lilly (Changchun, China); glucose, total cholesterol (TC), and triglyceride (TG) test kits were obtained from Beijing BHKT Clinical Reagent Co., Ltd. (Beijing, China); iodine [125I] insulin radioimmunoassay kit was purchased from Tianjin Nine Tripods Medical & Bioengineering Co., Ltd. (Tianjing, China). Other reagents were purchased from Beijing General Chemical Reagent Factory (Beijing, China). Western blot kits for NF-κB, and kits for TNF-α were from Wuhan Boster Biological Engineering Co., Ltd. (Wuhan, China) and Nanjing Jiancheng Biological Engineering Research Institute (Nanjing, China), respectively.
Six-week-old male Wistar rats were purchased from the Experimental Animal Center of Shandong University. The use of animals in these experiments received institutional animal care and use committee (IACUC) approval (approval No. SCU20090606). The animals were housed under standard laboratory conditions and maintained under controlled room temperature and humidity with a cycle of 12 hr of light and 12 hr of dark. All rats were randomly divided into two groups: normal control group and diabetic model group. The normal control group was fed with regular diet (8% fat, 68% carbohydrate, 24% protein, 320 kcal 100 g/L) and the others were fed with high-fat diet (60% fat, 20% carbohydrate, and 20% protein, 560 kcal 100 g/L). Eight weeks later, the diabetic model group were received intraperitoneal injection with a low dose of STZ (30 mg/kg) while the control group were given vehicle citrate buffer (pH 4.4) in a dose volume of 0.25 mL/kg, respectively. This diabetic model was similar to type 2 diabetes in human, where the high-fat diet intake initiated a state of insulin resistance and then the treatment of the relatively low dose of STZ established a relative insulin deficiency (11). The diabetic model group was further randomly divided into three groups: diabetic group, metformin-treated group, and aspirin-treated group. Metformin-treated group were administered to metformin (100 mg/kg/day) and aspirin-treated group were treated with aspirin (120 mg/kg/day) (12). Control group and diabetic control group were given the same amount of water. The rats were sacrificed after treatment of 8 weeks. The livers were perfused and collected in formalin for immunohistochemistry. The remaining liver tissues were fresh-frozen in liquid nitrogen for protein analysis.
Blood was sampled by intracardiac puncture. Plasma triglyceride (TG), cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) and free fatty acids (FFAs) concentrations were determined by using colorimetric assays. Plasma insulin was assayed by radioimmunoassay according to the instruction. The concentrations of serum TNF-α was measured by radioimmunoassay with an automatic biochemical analytic instrument. The marker of insulin resistance was evaluated by homeostasis model assessment estimate of insulin resistance (HOMA-IR) (13) as follows: fasting plasma insulin (µU/mL) × fasting glucose (mM/L)/22.5.
An equal liver sample from each rat (1 × 1 × 0.5 cm) was fixed in 40 g/L formaldehyde for 48 hr, embedded in paraffin, and sectioned. The 5 µm sections were stained with hematoxylin and eosin for general histopathological examination. Tissues were dissected and fixed in formalin overnight. Formalin-fixed tissues were sectioned and used for immunohistochemistry reveal NF-κB in hepatic tissues. Tissue sections of 5 µm were deparaffinized in xylene and rehydrated in ethanol followed by water and phosphate-buffered saline. Endogenous peroxidase was blocked by immersion in 3% hydrogen peroxide. The tissue sections were then incubated with NF-κB antibodies at a concentration of 5 µg/mL for 1 hr at room temperature. Control sections were incubated with phosphate-buffered saline containing normal goat serum without a primary antibody. Immunostaining was then detected with a streptavidin-biotin complex (SABC) kit and developed with diaminobenzidine tetrahydrochloride. The sections were counterstained with hematoxylin followed by light microscopy.
Total protein was extracted according to the manufacturer's instructions. For NF-κB, a KEYGEN nuclear-cytosol protein extraction kit was used. Western blot was performed according to the manufacturer's procedures. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (14) with a mini-slab apparatus. Sample of 50 µg protein was added to each lane. After polyacrylamide gel electrophoresis, the proteins were transferred onto nitrocellulose membranes. For protein signal detection, the samples were blocked with a solution of tris-buffered saline containing 5% fatfree milk and 0.1% Tween-20 (TBST) at room temperature for 1 hr, probed with anti-NF-κB antibody, and washed three times in TBST. The membranes were incubated with alkaline phosphatase-conjugated secondary antibody for 2 hr at room temperature and washed three times with TBST. Products were stained with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate and scanned and quantified using Pro-plus 5.0 image-processing system to determine the gray scale value.
All data were subjected to statistical analysis by using SPSS 16.0 statistical package. The data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by one way ANOVA. The number of inter-group differences were performed using Student-Newman-Keuls test. Relationship between two variables was analyzed by Pearson's correlation. P value of less than 0.05 was considered statistically significant.
The results of the present work showed development of insulin resistance in the diabetic group, which was expressed as a significant increase in HOMA-IR when compared to the control group (7.07 ± 1.88 vs 2.22 ± 0.26; P < 0.001). When compared to the control group, the diabetic group showed significant increase in serum levels of fasting glucose (9.82 ± 1.21 vs 4.78 ± 0.31; P < 0.001), insulin (15.95 ± 2.72 vs 10.43 ± 0.74; P < 0.001), FFAs (825.30 ± 93.39 vs 202.82 ± 85.43), TG (2.07 ± 0.28 vs 0.78 ± 0.29; P < 0.001) and TNF-α (75.30 ± 8.09 vs 25.74 ± 3.84; P < 0.001) (Table 1).
Aspirin-treated group showed significant reduction of the following studied parameters when compared to the diabetic group: insulin (11.74 ± 2.87 vs 15.95 ± 2.72, P < 0.001), HOMA-IR (4.85 ± 1.78 vs 7.07 ± 1.88, P < 0.001), FFAs (624.57 ± 98.67 vs 825.30 ± 93.39) and TNF-α (44.97 ± 4.67 vs 75.30 ± 8.09, P < 0.001), respectively. However, there is no significance difference in fasting glucose (9.04 ± 1.19 vs 9.82 ± 1.21, P = 0.353)
The metformin-treated group showed significant reduction of the following parameters when compared to the diabetic group: fasting glucose (7.66 ± 0.82 vs 9.82 ± 1.21, P < 0.001), insulin (13.89 ± 2.34 vs 15.95 ± 2.72, P < 0.001), HOMA-IR (4.79 ± 1.23 vs 7.07 ± 1.88, P < 0.001) and FFAs (713.35 ± 104.58 vs 825.30 ± 93.39), respectively. However, there was no significant change in TNF-α (75.75 ± 5.60 vs 75.30 ± 8.09, P = 0.754).
Hepatocytes, separated from the sinusoids, were large polyhedral cells with round nuclei in control group, (hematoxylin and eosin stain, × 75). Hepatocytes became larger; and red-stained granules within cytoplasm showed glycogen deposition in the other three groups. Hepatic sinusoids became slightly thinner (Fig. 1). Hepatocyte NF-κB was mainly expressed in the cytoplasm and weakly expressed in the nuclei in the control group. Hepatocyte NF-κB was expressed both in the cytoplasm and nuclei in the diabetic group and the metformin-treated group. But in the aspirin-treated group, hepatocyte NF-κB expression was reduced significantly and was seen rarely in the nuclei (Fig. 1). The positive expression rate of NF-κB was increased significantly in the diabetic group and metformin-treated group than in the control group. There was no difference in NF-κB-positive nuclei in the control group and aspirin-treated group (Table 2).
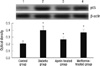
NF-κB p65 was not expressed or weakly expressed in normal control group. The positive expression rate of NF-κB p65 was significantly higher in diabetic and metformin-treated group than in normal control and aspirin-treated group. There were no significant differences between normal control and aspirin-treated group (Fig. 2).
In this study, we provided evidence that aspirin improves insulin resistance by inhibiting hepatic NF-κB activation and TNF-α level in streptozotocin-induced type 2 diabetic rats. Insulin resistance is not only an initiating pathogenic but also a major factor that promotes the development of type 2 diabetic. Overt diabetes is thought to be preceded by a long period of insulin resistance, during which sufficient insulin is produced to maintain normal or near-normal glucose tolerance. However, this chronic compensatory insulin hypersecretion to overcome tissue insensitivity can itself finally lead to pancreatic beta cell failure and overt hyperglycemia (4, 15). In the present study, the group of streptozotocin-induced type 2 diabetes rats showed significant increase in fasting plasma glucose, insulin, and HOMA-IR value when compared to the normal control group, which indicated development of insulin resistance.
Since Pickup et al. (16) first proposed diabetes is an inflammatory disease state in 1997, growing evidence has pointed to a correlation between various proinflammatory cytokines and insulin resistance/type 2 diabetes (17-19). Recent data have revealed that adipocyte-derived TNF-α, an inflammatory cytokine produced mainly by monocytes and macrophages was increased in the insulin resistant states of obesity and type 2 diabetes. In our study we also found that serum TNF-α was increased in diabetic group and positively correlated with insulin resistance. Thus, TNF-α has been recognized as an important mediator for insulin resistance by impairing insulin signaling. Specifically, TNF-α can decrease glucose uptake and utilization of peripheral tissues by targeting insulin signaling pathways and glucose transporter 4 (GLUT4). The mechanism of the decrease is due to stimulation of serine phosphorylation of IRS1 and IRS2, inhibiting tyrosine kinase activity of the insulin receptor and phosphatidylinositol-3-kinase (PI3K) signaling pathways (20).
NF-κB is a proinflammatory master switch that controls the production of a host of inflammatory markers and mediators, including TNF-α, IL-6, CRP, and PAI-1. Many studies suggest that NF-κB is closely related to the development of insulin resistance of type 2 diabetic (9, 10, 21). The mechanism of NF-κB causing insulin resistance has not studied thoroughly but studies have found that NF-κB can be activated by sustained high blood sugar (22). In addition, activation of the NF-κB induces the expression of many inflammatory cytokines such as TNF-α, IL-6, which are crucial factors in insulin resistance. Finally, TNF-α is not only induced by NF-κB, but also a strong activator of NF-κB. TNF-α binding induces activation of the IKK kinases, which regulates NF-κB transcriptional activity, finally leading to degradation of IRS, inhibition of tyrosine phosphorylation, and limit for signal transmission from the insulin receptor to PI3K (23). In our study we also found that TNF-α was increased accompanied by increased expression of hepatic NF-κB in the diabetic group. However, TNF-α was decreased and hepatic NF-κB expression was reduced after aspirin treatment. These suggest that NF-κB activation promotes TNF-α secretion and increased TNF-α level further stimulates the NF-κB activation, the two of which form a positive feedback loop and promote severe insulin resistance. After aspirin intervention, inhibition of NF-κB expression also reduced TNF-α level and improved the insulin resistance.
The liver is the key organ of insulin resistance in type 2 diabetes. Decreased hepatic insulin sensitivity may lead to increased hepatic gluconeogenesis, postprandial hyperinsulinemia and increased formation of triglycerides in the liver cells. In our study, we found that hepatocytes became larger and showed glycogen deposition in the cytoplasm. Immunohistochemistry showed hepatocyte NF-κB was expressed both in the cytoplasm and nuclei in the diabetic group and the metformin-treated group. Western blot showed the positive expression rate of NF-κB p65 was significantly higher in diabetic and metformin-treated group than in control group. These suggest that an up-regulation of hepatocyte NF-κB is strongly associated with systemic insulin resistance, which is consistent with pervious two studies (9, 10), indicating that IKK-β and NF-κB play important roles in the progress of hepatic insulin resistance. These two findings also indicate that lipid accumulation in the liver leads to hepatic 'inflammation' through NF-κB activation and downstream cytokine production, upregulates TNF-α production and secretion. TNF-α works locally through paracrine and/or endocrine mechanisms to activate TNF-α signaling in the liver. Given that the link between insulin resistance and inflammation, limiting inflammation and reducing levels of inflammatory markers may be a promising therapeutic strategy. Aspirin, one of the most widely used medications in the world, has been used to analgesic, antipyretic, and anti-inflammatory for more than 100 yr. In recent years, aspirin was found not only to have anti-platelet aggregation and prevent cardiovascular diseases, but also have a role in the reversal of obesity- and diet-induced insulin resistance (24).
In our study, we found that the aspirin-treated group showed significant reduction of serum insulin and HOMA-IR, indicating aspirin can improve insulin resistance. We also found that aspirin had significant therapeutic effect on improving blood lipids, although the hypoglycemic effect was not obvious. The mechanism of aspirin improving insulin resistance has not been studied clearly. Previous studies showed that aspirin can prevent NF-κB activation by inhibiting IKK activation and dissociation of NF-κB from IκB (4), so that NF-κB cannot control the downstream expression of inflammatory cytokines, which reduce insulin resistance. In our study we also found that hepatocyte NF-κB was expressed both in the cytoplasm and nuclei in the diabetic group. But in the aspirin-treated group, hepatocyte NF-κB expression was reduced significantly and was seen rarely in the nuclei. TNF-α levels were also decreased compared to diabetic groups. These suggest that aspirin improves insulin resistance by inhibiting hepatic NF-κB activation and TNF-α level.
In conclusion, the inflammatory pathways hold a substantial part in insulin resistance of type 2 diabetes mellitus. Therefore, diabetes therapy should not only focus on glycemic control but also on the underlying mechanism to improve insulin resistance. Aspirin, an old drug for new use, offers unique approaches for the treatment of type 2 diabetes because of its insulin-sensitizing and anti-inflammatory properties.
Figures and Tables
Fig. 1
HE staining and NF-κB expression of liver tissues from four studied groups. (A) HE staining in NC group. (B) HE staining in diabetic group. (C) HE staining in aspirin-treated group. (D) HE staining in metformin-treated group. (E) NF-κB expression in NC group. (F) NF-κB expression in diabetic group. (G) NF-κB expression in aspirin-treated group. (H) NF-κB expression in metformin-treated group (× 400).

Fig. 2
Western blot analyses of NF-κB (p65) of liver tissues from four studied groups. Lane 1, control group; Lane 2, diabetic group; Lane 3, aspirin-treated group; Lane 4, metformin-treated group. *P < 0.001, compared to control group; †P < 0.001, compared to diabetic group.
AUTHOR SUMMARY
Effect of Aspirin on the Expression of Hepatocyte NF-κB and Serum TNF-α in Streptozotocin-Induced Type 2 Diabetic Rats
Xiaodong Sun, Fang Han, Junling Yi, Lina Han and Ben Wang
We hypothesized that aspirin might improve insulin resistance in type 2 diabetes by inhibiting hepatic NF κB activation and serum TNF-α. Adult male Wistar rats were randomly divided into four groups: control, untreated diabetic, diabetic treated with metformin, and diabetic treated with aspirin. Diabetes was induced by high-fat feeding and a low dose of streptozotocin. Aspirin did not lowered fasting glucose level but significantly reduced hepatic NF-κB expression and serum TNF-α level with improved insulin resistance. The relevant analysis showed positive correlation between the expression of HOMA-IR and NF-κB, HOMA-IR and serum TNF-α. It is suggested that aspirin improves insulin resistance by inhibiting hepatic NF-κB activation and TNF-α level in streptozotocin-induced type 2 diabetic rats. Aspirin, an old drug for new use, might offer unique approaches for the treatment of type 2 diabetes.
References
1. Virally M, Blicklé JF, Girard J, Halimi S, Simon D, Guillausseau PJ. Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectives. Diabetes Metab. 2007. 33:231–244.
2. Spellman CW. Pathophysiology of type 2 diabetes: targeting islet cell dysfunction. J Am Osteopath Assoc. 2010. 110:S2–S7.
3. Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003. 27:Suppl 3. S53–S55.
4. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKK-β. Science. 2001. 293:1673–1677.
5. Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002. 109:1321–1326.
6. Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000. 102:42–47.
7. Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001. 9:414–417.
8. Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH. Atherosclerosis Risk in Communities Study. Fasting plasma free fatty acids and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004. 27:77–82.
9. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005. 11:183–190.
10. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005. 11:191–198.
11. Wang HJ, Jin YX, Shen W, Neng J, Wu T, Li YJ, Fu ZW. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac J Clin Nutr. 2007. 16:Suppl 1. 412–417.
12. Abdin AA, Baalash AA, Hamooda HE. Effects of rosiglitazone and aspirin on experimental model of induced type 2 diabetes in rats: focus on insulin resistance and inflammatory markers. J Diabetes Complications. 2010. 24:168–178.
13. Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002. 106:679–684.
14. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.
15. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004. 24:816–823.
16. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997. 40:1286–1292.
17. Park K, Steffes M, Lee DH, Himes JH, Jacobs DR Jr. Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. 2009. 52:2337–2344.
18. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010. 72:219–246.
19. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010. 185:1836–1845.
20. Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003. 321:1–15.
21. Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009. 296:H1850–H1858.
22. Soriano FG, Virág L, Szabó C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly (ADP-ribose) polymerase activation. J Mol Med. 2001. 79:437–448.
23. Wunderlich FT, Luedde T, Singer S, Schmidt-Supprian M, Baumgartl J, Schirmacher P, Pasparakis M, Brüning JC. Hepatic NF-kappa B essential modulator deficiency prevents obesity-induced insulin resistance but synergizes with high-fat feeding in tumorigenesis. Proc Natl Acad Sci USA. 2008. 105:1297–1302.
24. Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001. 108:437–446.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download