Abstract
Prediction of malignancy or invasiveness of branch duct type intraductal papillary mucinous neoplasm (Br-IPMN) is difficult, and proper treatment strategy has not been well established. The authors investigated the characteristics of Br-IPMN and explored its malignancy or invasiveness predicting factors to suggest a scoring formula for predicting pathologic results. From 1994 to 2008, 237 patients who were diagnosed as Br-IPMN at 11 tertiary referral centers in Korea were retrospectively reviewed. The patients' mean age was 63.1 ± 9.2 yr. One hundred ninty-eight (83.5%) patients had nonmalignant IPMN (81 adenoma, 117 borderline atypia), and 39 (16.5%) had malignant IPMN (13 carcinoma in situ, 26 invasive carcinoma). Cyst size and mural nodule were malignancy determining factors by multivariate analysis. Elevated CEA, cyst size and mural nodule were factors determining invasiveness by multivariate analysis. Using the regression coefficient for significant predictors on multivariate analysis, we constructed a malignancy-predicting scoring formula: 22.4 (mural nodule [0 or 1]) + 0.5 (cyst size [mm]). In invasive IPMN, the formula was expressed as invasiveness-predicting score = 36.6 (mural nodule [0 or 1]) + 32.2 (elevated serum CEA [0 or 1]) + 0.6 (cyst size [mm]). Here we present a scoring formula for prediction of malignancy or invasiveness of Br-IPMN which can be used to determine a proper treatment strategy.
Intraductal papillary mucinous neoplasm (IPMN) is a tumor characterized by dilatation of the main pancreatic duct with mucin; it has distinct malignant potential (1). In recent times, the clinical and pathological characteristics have been elucidated, and several treatment guidelines have been suggested (2-4). IPMNs involving the main pancreatic duct have a relatively high risk of progression to malignancy and invasive carcinoma, so International Consensus Guidelines recommend surgical resection of IPMN involving the main pancreatic duct (3-6).
Branch duct type IPMN is known to carry a lower risk of malignancy compared to main duct IPMN. Hence, there are many different opinions about the necessity of surgical resection and the timing of surgery (4). Furthermore, controversy still exists with regard to when to operate and when to observe. It is certain that malignant IPMN, especially invasive IPMN, has poorer prognosis compared to benign and non-invasive IPMN (1, 4, 7). When selecting a treatment for branch duct IPMN, we consider several factors, including tumor behavior and the patient's condition. However, the most important factor affecting our treatment decision may be tumor malignancy. Recently published papers have demonstrated similar concepts regarding branch duct type IPMN (4, 8, 9). In the clinical setting, these results gave us a lot of help in determining the surgical management, but predicting the risk for an individual patient was more important and valuable than enumerating the overall risk using statistical analysis. Hence, a formula for predicting the malignancy risk of an individual patient would be greatly helpful.
The aims of this study were to investigate the predictors of malignant and invasive IPMN, and to construct a formula to calculate the probability of malignancy and invasiveness. By using this method, we hoped to suggest new surgical guidelines for branch duct type IPMN that does not involve the main pancreatic duct.
We retrospectively collected the data for IPMN cases registered between January 1994 and September 2008. Eleven tertiary medical centers with specialized biliary-pancreatic surgeons, gastrointestinal radiologists, and gastrointestinal pathologists participated in this study.
We designed standardized case-report forms containing fields for IPMN clinical, radiological, and pathological characteristics and survival data. A standard protocol for interpreting radiological and pathological data was made by experienced radiologists and pathologists at Seoul National University Hospital.
For standardized radiological information, images from cross-sectional studies (contrast-enhanced computed tomography [CT] and magnetic resonance imaging [MRI]) and pancreatography (magnetic resonance cholangiopancreatography [MRCP] or endoscopic retrograde cholangiopancreatography [ERCP]) were used to classify the type of IPMN. Standardized criteria included IPMN morphology (main, branch, or mixed), anatomic location of the main lesion, distribution, multifocality, presence of mural nodule, lesion size, septation, wall thickening, calcification, and parenchymal atrophy. Analyses of these factors were based mainly on CT, and information from other imaging methods was used to support the information gained from CT.
Branch duct type IPMN was defined as a cystic dilatation of a branch pancreatic duct or a pancreatic cyst communicating with the pancreatic duct without main duct dilatation. The term 'without main duct dilatation' indicated a maximal main pancreatic duct diameter of 5 mm or less. This was thought to be the most restricted and well-represented criteria for branch duct type IPMN (3, 5, 10-12). Main duct type IPMN was radiologically defined as a main pancreatic duct dilated > 5 mm. Mixed type IPMN was defined as radiologic evidence of both main and branch duct type IPMN (3, 5, 10-12). Wall thickening was considered to be present when the wall was ≥ 2 mm in maximum thickness and covered at least one-third of the cystic lesion or the dilated pancreatic duct. Pancreatic parenchymal atrophy was defined as a situation in which the ratio of the diameter of the main pancreatic duct to the width of the pancreatic parenchyma at the same location was greater than 0.5 (12). A mural nodule was defined as a soft tissue nodule attached to the lesion wall, or as a septum in the pancreatic cystic lesion or the dilated main pancreatic duct. Mural nodule status only was applied in this study. Mural nodule size was not investigated, because CT is not the gold standard for measuring mural nodule size (12).
During the above-mentioned period, a total of 388 patients preoperatively diagnosed with IPMN and cystic dilatation of a branch duct underwent surgical resection. Pathological confirmation was performed in all 11 medical centers. Patients with main ducts dilated to larger than 5 mm (n = 151) were excluded from this study. Thus, a total of 237 patients were eligible for this study. We analyzed the medical records, results of preoperative laboratory and radiologic examination and postoperative pathologic examination, and follow-up data.
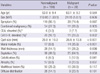
The study subjects consisted of 137 men and 100 women of mean age 63.1 yr (range, 38-83 yr). There were 148 (62.4%) symptomatic patients. Frequently manifested symptoms and signs included abdominal pain, weight loss, jaundice, diabetes mellitus, and pancreatitis. Elevated serum carcinoembryonic antigen (CEA, > 5 ng/mL) was identified in 9 (3.8%) patients, and elevated serum carbohydrate antigen 19-9 (CA 19-9, > 37 U/mL) was detected in 31 (13.1%) patients. The mean cystic tumor size was 28.1 ± 18.0 mm. Thirty-one (13.1%) patients had mural nodules on CT (Table 1). Combined extrapancreatic malignancies were detected in 65 (27.4%) patients. Stomach cancer was the most common malignancy (26 patients, 11.0%), followed by colorectal cancer (14 patients, 5.9%), other hepatobiliary-pancreatic cancer (12 patients, 5.1%), and urologic cancer (5 patients, 2.1%). Among the various operative procedures, pancreaticoduodenectomy (including pylorus-preserving procedures) was most common (108 [45.6%] patients). For limited resection, duodenum-preserving resection of the head of the pancreas, pancreatic head resection with segmental duodenectomy, median pancreatectomy, and excision were performed in 4 (1.7%), 3 (1.3%), 12 (5.1%), and 14 (5.9%) patients, respectively. Pathologic examination according to the AJCC 6th edition manual showed malignant IPMN (carcinoma in situ or invasive IPMC) in 39 (16.5%) patients and invasive IPMN in 26 (11.0%) patients. Extrapancreatic tumor extension (pT3) was identified in 8 (30.8%) patients with invasive IPMN, and metastatic lymph nodes were identified in 3 (11.5%) patients with invasive IPMN (n = 26) (Table 2).
Differences between categorical variables were analyzed using the chi-squared test and Fisher's exact test, while the Student t-test and Mann-Whitney U test were used for comparisons among continuous variables. The statistical correlation between outcomes and categorical/continuous factors was determined using logistic regression analysis. Statistical significance was defined as P < 0.05 (two-sided P values).
Parameters identified by univariate analysis with P < 0.05 were entered into multivariate analysis to identify independent malignant and invasive predicting factors.
Receiver-operator characteristic (ROC) curves were used to determine optimal score cutoff levels for the prediction of malignant and invasive IPMN. All analyses were carried out using SPSS version 15.0 for Windows (SPSS, Chicago, IL, USA).
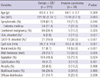
The subjects were divided into two groups: nonmalignant and malignant IPMN. The nonmalignant IPMN group (n = 198) included patients with adenomas and borderline tumors, and the malignant IPMN group (n = 39) included patients with carcinoma in situ and invasive IPMC. On univariate analysis, elevated CA 19-9 (P = 0.002), larger cyst size (P < 0.001), the presence of mural nodules (P < 0.001), wall thickening (P = 0.036), calcification (P = 0.041), and parenchymal atrophy (P = 0.037) were statistically significant (Table 3). When multivariate analysis was performed for these factors, larger cyst size (P = 0.021) and the presence of mural nodules (P = 0.001) remained significant (Table 4).
Adenomas, borderline tumors, and carcinoma in situ were classified as noninvasive IPMN. Diabetes mellitus (P = 0.036), elevated serum CEA (P = 0.027) or CA 19-9 (P < 0.001), larger cyst size (P = 0.001), presence of mural nodules (P < 0.001), wall thickening (P = 0.005), and septation (P = 0.024) were significant variables on univariate analysis (Table 5). On multivariate analysis, elevated CEA (P = 0.043), larger cyst size (P = 0.034), and mural nodules (P < 0.001) were statistically significant (Table 6).
The scoring rule was developed using a logistic regression model with the following form:
Logit (Y = 1) = ln (P(Y = 1)/[1-P(Y = 1)]) = b0 + b1X1 + b2X2 + ... + bnXn,
where Y = 1 indicates malignant or invasive IPMN, and ln is the natural logarithm. The formula shows that the predictors (X1-Xn) are linear, and on an additive scale related to the logit (Y = 1), with relative weights b1-b2; that is, the regression coefficients. This means that, for a particular subject with a certain X1-Xn profile, the regression coefficients multiplied by the patient's X values may simply be added to obtain the logit and, by logistic transformation, the probability of Y = 1. Commonly, however, the regression coefficients are multiplied by a number (for instance, 10) and rounded to the nearest value to easily obtain applicable scores per predictor (13).
First, this formula was applied to malignancy-predicting factors. The regression coefficient of cyst size was 0.045, and that of mural nodule presence was 2.237. These coefficients were multiplied by 10 and rounded off. Then the formula was constructed as follows:
Malignancy-predicting score = 22.4 (presence of mural nodule [0 or 1]) + 0.5 (size of cyst [mm]).
The malignancy-predicting scores were calculated on whole subjects based on this scoring formula, and optimal cutoff values were determined using a receiver-operator characteristic (ROC) curve (Fig. 1). The optimal cutoff value was determined to be 14, and subjects were divided into two groups: 14 points or more, and less than 14 points. Significant differences (P < 0.001) were identified between the two groups, with sensitivity of 71.8%, specificity of 60.0%, and accuracy of 61.6%.
These results indicated that branch duct type IPMN with mural nodule should be regarded as malignant IPMN itself. If preoperative CT does not identify mural nodule, cyst size greater than 28 mm should be regarded as indicating malignant IPMN.
Invasiveness-predicting scores were obtained in the same way. The regression coefficient of elevated CEA was 3.216, that of cyst size was 0.061, and that of mural nodule presence was 3.660. The scoring formula was as follows:
Invasiveness- predicting score = 36.6 (presence of mural nodule [0 or 1]) + 32.2 (elevated serum concentration of CEA [0 or 1]) + 0.6 (size of cyst [mm]).
After calculating the invasive-predicting scores, the best cutoff value was determined to be 21 points by ROC curve. Based on a 21-point cutoff, the two groups were shown to be significantly different (P < 0.001) with sensitivity of 76.9%, specificity of 72.5%, and accuracy of 73.1% (Fig. 2). These results indicated that patients with mural nodule or elevated serum CEA should be considered to have invasive IPMN. If the patient had no evidence of mural nodules or elevated serum CEA, cyst size greater than 35 mm was regarded as indicating the presence of invasive IPMN.
Ever since the first report of Ohashi in 1982 (14), asymptomatic cystic tumors of the pancreas have been detected with increasing frequency. This has resulted in a dramatic increase in the diagnosis of IPMN at specialized centers. Increasing frequency of diagnosis is specifically attributed to improved diagnostic techniques, as well as the accumulation of knowledge concerning the clinicopathologic characteristics and natural history of specific IPMN subtypes.
The management of branch duct type IPMN has proved to be complicated. Because branch duct type IPMN has a relatively lower risk of malignant or invasive IPMN development, controversy exists regarding the need for surgical resection. The International Consensus Guidelines have suggested an algorithm for surgical management, which is based on cyst size, patient symptoms, and "high risk stigmata" (mural nodules, positive cytology). Surgical resection 1) is not recommended in patients with branch duct type IPMN < 1 cm, 2) but is recommended in patients with IPMN in the range of 1 to 3 cm if symptoms, mural nodules, or positive cytology are present, and 3) is recommended in patients with IPMN > 3 cm based on size alone (3, 5). These recommendations were based mainly on cyst size, symptoms, the presence of mural nodule, and positive cytology.
Our study showed similar results in that the presence of mural nodule and larger cyst size had a statistically significant correlation with malignant and invasive IPMN (5, 9, 15, 16). These are the independent criteria used to determine if surgery, and specifically radical resection, is needed. Because these factors are included into scoring formula, each factor has a statistical significance in itself as well as with the others. So, scoring formula is seemed to be complex, but each acts as an independent factor. Thus, in absence of mural nodule, the size of IPMN directly correlated with the risk of malignancy/invasiveness. If preoperative workup reveals the presence of mural nodules or cyst size ≥35 mm, the lesion should be considered to represent invasive IPMN, and radical resection should be performed.
Elevated serum CEA is another independent predictor of invasiveness. Considering that other reports have noted elevated serum CEA as a predictor of malignant IPMN, this finding alone is enough to indicate the diagnosis (17, 18).
The results of the present study differ from those of other investigators' reports, which have indicated that branch duct type IPMN, especially smaller than 30 mm in size, is sufficient cause to apply limited resection of the pancreas (8, 19-21). Only 12 of 32 patients with main duct involvement had cysts larger than 30 mm in size. Twenty (62.5%) patients with Br-IPMN smaller than 30 mm had main duct involvement on pathologic examination. We did not investigate differences in clinical outcomes or prognoses according to the presence of main duct involvement (including recurrence and survival), so we do not know the clinical importance of this finding. However, if limited resection is intended preoperatively for nonmalignant IPMN, the range of resection must be determined very carefully because IPMN may involve the main pancreatic duct. The clinical importance of main duct involvement will be investigated in the future.
In order to determine the necessity of radical resection, the likelihood of malignancy and invasiveness must be determined on preoperative workup. This is essential to avoid making a diagnosis of malignant or invasive IPMN at the postoperative period. However, until this time, a formula to predict the risk of malignancy and invasiveness for individual patients has not been established, and it has been difficult to determine treatment based on preoperative predictions. However, the scoring formula based on this study corresponds with clinical data and matches a large number of previous papers (7, 9, 22-24).
Many factors must be considered when determining whether to operate or observe patients with branch duct type IPMN, from general surgical considerations to IPMN-specific ones. Clinicians must be attentive to the presence of mural nodule, the elevation of serum CEA, and the size of any cysts. If these actions are undertaken, the scoring formula for this study can be applied in decision-making. Additional studies will help confirm the validity of our findings. Clinicians should then be able to arrive at a firm consensus on the appropriate management of branch duct type IPMN.
Figures and Tables
 | Fig. 1Receiver operating characteristic (ROC) curve between the malignancy-predicting score and malignancy. Considering sensitivity and specificity, 14 points was the optimal cutoff value. |
 | Fig. 2Receiver operating characteristic (ROC) curve of calculated score, related to invasiveness. In distinguishing noninvasive IPMN and invasive IPMN, 21 was the most reliable cutoff value. |
AUTHOR SUMMARY
Determination of Malignant and Invasive Predictors in Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Suggested Scoring Formula
Dae Wook Hwang, Jin-Young Jang, Chang-Sup Lim, Seung Eun Lee, Yoo-Seok Yoon, Young Joon Ahn, Ho-Seong Han, Sun-Whe Kim, Sang Geol Kim, Young Kook Yun, Seong-Sik Han, Sang-Jae Park, Tae Jin Lim, Koo Jung Kang, Mun Sup Sim, Seong Ho Choi, Jin Seok Heo, Dong Wook Choi, Kyung Yul Hur, Dong-Shik Lee, Sung-Su Yun, Hong-Jin Kim, Chul Kyoon Cho, Hyun Jong Kim, Hee Chul Yu, Baik Hwan Cho and In-Sang Song
In order to determine the necessity of radical resection for branch duct type intraductal papillary mucinous neoplasms (IPMN), the likelihood of malignancy and invasiveness must be determined on preoperative workup. This is essential to avoid making a diagnosis of malignant or invasive IPMN at the postoperative period. However, until this time, a formula to predict the risk of malignancy and invasiveness for individual patients has not been established. The scoring formula based on this study corresponds with clinical data and matches a large number of previous papers. We suggest to evaluate the presence of mural nodule, the elevation of serum CEA, and the size of any cysts. If these actions are undertaken, the scoring formula for this study can be applied in decision-making and be helpful for determining surgical management.
References
1. Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004. 239:788–797.
2. Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Kloppel G, Luttges J, Memoli VA, Tosteson TD, Yanagisawa A, Wilentz R, Zamboni G. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005. 31:344–349.
3. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006. 6:17–32.
4. Jang JY, Kim SW, Lee SE, Yang SH, Lee KU, Lee YJ, Kim SC, Han DJ, Choi DW, Choi SH, Heo JS, Cho BH, Yu HC, Yoon DS, Lee WJ, Lee HE, Kang GH, Lee JM. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008. 15:199–205.
5. Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, Akisik FM, Sherman S, Pitt HA, Lillemoe KD. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007. 246:644–651.
6. Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004. 239:678–685.
7. Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, Han JK, Kim WH, Lee YJ, Kim SC, Han DJ, Kim YI, Choi SH, Cho BH, Yu HC, Yoon DS, Lee WJ, Lee KB, Kim YC, Lee KS, Kim MW, Kim HJ, Park YH. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005. 12:124–132.
8. Paik KY, Choi SH. Experience of limited pancreatic head resection for management of branch duct intraductal papillary mucinous neoplasm in a single center. World J Gastroenterol. 2009. 15:2904–2907.
9. Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2010. doi: 10.1007/s00423-010-0674-6.
10. Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, Sandrasegaran K, Akisik F, Howard TJ, Nakeeb A, Zyromski NJ, Lillemoe KD. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008. 12:101–109.
11. Sahani DV, Kadavigere R, Blake M, Fernandez-Del Castillo C, Lauwers GY, Hahn PF. Intraductal papillary mucinous neoplasm of pancreas: multi-detector row CT with 2D curved reformations--correlation with MRCP. Radiology. 2006. 238:560–569.
12. Chiu SS, Lim JH, Lee WJ, Chang KT, Oh DK, Lee KT, Lee JK, Choi SH. Intraductal papillary mucinous tumour of the pancreas: differentiation of malignancy and benignancy by CT. Clin Radiol. 2006. 61:776–783.
13. Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol. 2002. 55:1054–1055.
14. Ohashi K, Murakami Y, Murayama M, Taketoshi T, Ohta T, Ohashi I. Four cases of mucin-producing cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982. 20:348–351.
15. Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancre as: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001. 96:1429–1434.
16. Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006. 186:687–695.
17. Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Namikawa T, Okamoto K, Okamoto N, Kosaki T, Onishi S, Araki K. Clinicopathological features and medical management of intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol. 2006. 21:462–467.
18. Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, Goulet RJ Jr, McHenry L, Sherman S, Lehman GA, Cramer H, Madura JA. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003. 138:610–617.
19. Kuroki T, Tajima Y, Tsutsumi R, Mishima T, Kitasato A, Adachi T, Kanematsu T. Inferior branch-preserving superior head resection of the pancreas with gastric wall-covering method for intraductal papillary mucinous adenoma. Am J Surg. 2006. 191:823–826.
20. Yamao K, Ohashi K, Nakamura T, Suzuki T, Shimizu Y, Nakamura Y, Horibe Y, Yanagisawa A, Nakao A, Nimuara Y, Naito Y, Hayakawa T. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology. 2000. 47:1129–1134.
21. Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002. 137:1274–1278.
22. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007. 56:1086–1090.
23. Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003. 90:1244–1249.
24. Serikawa M, Sasaki T, Fujimoto Y, Kuwahara K, Chayama K. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol. 2006. 40:856–862.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download