Abstract
Cytokines such as interleukin 10 (IL10) may play an important role in the process of inflammation. The aim of this study was to analyze the association between IL10, IL10RA and IL10RB single nucleotide polymorphisms (SNPs), and benign prostate hyperplasia (BPH) in Korean population. All patients with BPH were divided into two groups according to international porostate symptom score (IPSS), prostate specific antigen (PSA) level, Qmax, and prostate volume. We selected two IL10 SNPs (rs1518111 and rs1554286), three IL10RA SNPs (rs2256111, rs4252243, and rs2228054), and two IL10RB SNPs (rs999788 and rs2834167). Genotypes of seven SNPs were determined through direct sequencing. The G/G genotype of IL10RB polymorphism (rs2834167) was associated with a high PSA level compared with the A/G + A/A genotypes (P = 0.009). Of IL10 SNP, the A/A genotype of rs1518111 and T/T genotype of rs1554286 were associated with small prostate volume, respectively (P = 0.011, P = 0.014). Moreover, the T/T genotype of IL10RB polymorphism (rs999788) was associated with high prostatic volume compared with the T/C + C/C genotypes (P = 0.033). The linkage disequilibrium (LD) blocks were formed in IL10 and IL10RA. However, haplotypes in the LD block were not associated with BPH. It is concluded that there is a strong association between the IL10 and IL10RB SNPs, and BPH in Korean population.
Benign prostatic hyperplasia (BPH) is a common disease and occurs in about one third of men in their sixties. In Korea, the overall prevalence of BPH was 40%, and this result suggests an increasing tendency of BPH prevalence (1). BPH is a complex disease from multiple etiologies and pathogenesis point of view. Recently, several reports revealed that BPH is related with immune-mediated inflammatory response (2, 3). Experimental investigations using prostatitis in mice and rats suggested autoimmune responses and genetic background as etiologic factors (4). Chronic inflammation has been documented for years in BPH, and it becomes evident as a major factor in disease initiation and progression (5).
Cytokines such as interleukin 10 (IL10) and interleukin 2 (IL2) have been found in prostate secretion fluids of chronic prostatitis (6). These cytokines maybe play an important role in the process of inflammation. IL10, which is anti-inflammatory cytokine opposing the inflammatory reaction, leads to increase levels of the regulatory cytokine (IL2) (6). IL10 is produced primarily by monocytes and to a lesser extent by lymphocytes. IL10 is capable of inhibiting synthesis of pro-inflammatory cytokines such as IL2, interleukin 3, tumor necrosis factor alpha and interferon alpha made by macrophages and type 1 T helper cells. The IL10 gene is located in chromosome 1 and consists of five exons. IL10 has been shown to interact with interleukin 10 receptor, alpha (IL10RA) and IL10 receptor, beta (IL10RB).
Single nucleotide polymorphism (SNP) may regulate the biosynthesis, activations, and inactivation of genes and could influence the pathogenesis of disease initiation and progression. Genetic strategy such as polymorphism has been used to investigate pathogenesis of BPH. Several polymorphisms have been reported to have positive associations with prostatic growth (7). The aim of this study was to analyze the association between IL10, IL10RA and IL10RB SNPs, and BPH in Korean population.
The present study consisted of 233 patients with BPH and 214 age-matched normal healthy patients. The patients with BPH were enrolled from Kyung Hee University Hospital between January 2002 and December 2008. All healthy control patients underwent screening and had a normal serum prostate-specific antigen (PSA) level (< 4 ng/mL). The patient with BPH complained of moderate or severe lower urinary tract symptoms.
Lower urinary tract symptoms were quantified using international prostate symptom score (IPSS). Uroflowmetry was done to measure peak urinary flow rate (Qmax) for all patients. Serum PSA levels were checked in all BPH patients. The patients with a serum PSA level more than 4 ng/mL underwent transrectal ultrasound-guided prostate biopsy to rule out prostate cancer. Prostate size was assessed with transrectal ultrasound. Exclusion criteria for this study were prostate cancer, neurogenic bladder, urinary tract infection, uncontrolled diabetes mellitus, and cardiovascular disease. All patients with BPH were divided into two groups according to several multicenter studies; low (0-19) and high (≥ 20) IPSS group, low (< 1.5 ng/mL) and high (≥ 1.5 ng/mL) PSA level, low (< 10 mL/sec) and high (≥ 10 mL/sec) Qmax, and small (< 30 mL) and large (≥ 30 mL) prostate volume (8, 9). Genomic DNA was extracted from blood samples collected in EDTA using the Qiagen DNA Extraction kit (Qiagen, Tokyo, Japan).
We selected two IL10 SNPs (rs1518111 and rs1554286), three IL10RA SNPs (rs2256111, rs4252243, and rs2228054), and two IL10RB SNPs (rs999788 and rs2834167) with greater than 0.3 heterozygosity among SNPs located in the exon and promoter region (http://www.ncbi.nlm.nih.gov/SNP, BUILD 130) for analysis (Fig. 1).
Genotypes of two IL10 SNPs (rs1518111 and rs1554286), three IL10RA SNPs (rs2256111, rs4252243, and rs2228054), and two IL10RB SNPs (rs999788 and rs2834167) were determined through direct sequencing. Genomic DNA was amplified using PCR primers (Table 1). PCR consisted of 35 cycles at 94℃ for 30 sec, 58℃ for 30 sec, 72℃ for 1 min, and 1 cycle at 72℃ for 7 min to terminate the reaction. For automated direct sequencing, the PCR products were amplified using internal forward and reverse primers and a PRISM BigDye Terminator v3.1 Cycle sequencing Kit. The DNA samples containing extension products were added to Hi-Di formamide (Applied Biosystems, Foster City, CA, USA). The mixture was incubated at 95℃ for 5 min, followed by 5 min on ice, and then analyzed by the ABI Prism 3730XL DNA analyzer (Applied Biosystems). Sequence data were analyzed using SeqManII software (v2.3; DNAATAR Inc., Madison, WI, USA).
We analyzed genetic data in control and BPH subjects. SNPStats (http://bioinfo.iconcologia.net/index.php), HelixTree (Golden Helix Inc., MT, USA), and SNPAnalyzer (ISTECH Inc., Goyang, Korea) software were used to analyze genetic data. The Hardy-Weinberg equilibrium (HWE) was assessed by SNPStats in control and BPH cases. Multiple logistic regression models (codominant, dominant and recessive model) were used to calculate odds ratio (OR), 95% confidence interval (CI), and corresponding P values with Bonferroni correction, while controlling for age as a covariable (10). A linkage disequilibrium (LD) block of polymorphisms was tested using Haploview version 4.02 (11). A P < 0.05 was considered significant.
The clinical characteristics of the study subject with BPH at baseline were given in Table 2. The median age of the BPH subjects was 66.0 yr. A total of 233 BPH subjects and 214 age-matched control subjects were genotyped to analyze associations between SNPs and BPH.
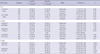
Genotype distributions of SNPs in this study were in Hardy-Weinberg equilibrium in control subjects (IL10 SNPs [rs1518111, P = 0.64 and rs1554286, P = 0.54], IL10RA SNPs [rs2256111, P = 0.18; rs4252243, P = 1.00; and rs2228054, P = 0.51], and IL10RB SNPs [rs999788, P = 0.49 and rs2834167, P = 0.34]). Genotype distributions of seven SNPs for control and BPH subjects are analyzed. There were no associations between control and BPH subjects, maybe, due to high prevalence in Korean population (IL10 SNPs [rs1518111, P = 0.70 and rs1554286, P = 0.69], IL10RA SNPs [rs2256111, P = 0.91; rs4252243, P = 0.26; and rs2228054, P = 0.83], and IL10RB SNPs [rs999788, P = 0.22 and rs2834167, P = 0.80]). The genotype distributions of seven SNPs were analyzed according to low (0-19) and high (≥ 20) IPSS scores. No significant associations were found between IPSS score (IL10 SNPs [rs1518111, P = 0.64 and rs1554286, P = 0.54], IL10RA SNPs [rs2256111, P = 0.18; rs4252243, P = 1.00; and rs2228054, P = 0.51], and IL10RB SNPs [rs999788, P = 0.49 and rs2834167, P = 0.34]). In allele analysis, there were no significant differences according to IPSS score. Table 3 shows genotype distributions of seven SNPs for low (< 1.5 ng/mL) and high (≥ 1.5 ng/mL) PSA level. The T/T genotype of IL10RB polymorphism (rs999788) was associated with a high PSA level compared with the T/C + C/C genotypes (95% CI 0.28-0.95, P = 0.031). Moreover, the G/G genotype of IL10RB polymorphism (rs2834167) was associated with a high PSA level compared with the A/G + A/A genotypes (95% CI 0.23-0.83, P = 0.009). In allele analysis, however, there were no significant differences. The genotype distributions of SNPs were analyzed according to low (< 10 mL/sec) and high (≥ 10 mL/sec) Qmax. There were no significant associations between low and high Qmax. In allele analysis, there were no significant differences according to Qmax.
There were significant associations between IL10 and IL10RB SNPs, and prostatic volume in Table 4. Of IL10 SNP, rs1518111 (95% CI 1.01-2.31, P = 0.044 in codominant model, 95% CI 1.17-3.34, P = 0.011 in dominant model) and rs1554286 (95% CI 1.00-2.30, P = 0.047 in codominant model, 95% CI 1.16-3.32, P = 0.014 in dominant model) were associated with prostatic volume. The G allele of rs1518111 and the C allele of rs1554286 were found to be significantly associated with large prostatic volume and a risk factor for increased prostatic volume in present study. Moreover, the T/T genotype of IL10RB polymorphism (rs999788) was associated with high prostatic volume compared with the T/C + C/C genotypes (95% CI 0.30-0.96, P = 0.033). In allele analysis, however, there were no significant differences according to prostatic volume.
Seven SNPs were analyzed for LD and haplotypes using Haploview (version 4.2) according to IPSS score, serum PSA level, Qmax, and prostatic volume. The LD blocks were formed in IL10 and IL10RA (Fig. 2). The LD block of IL10 gene consisted of rs1554286 and rs1518111. The LD block of IL10RA gene consisted of rs2256111 and rs2228054. However, haplotypes in the LD block of IL10 and IL10RA were not associated within BPH subjects based on prostatic volume (Table 5).
Cytokines play multiple roles in both immune disorders and many complex diseases. Inflammatory processes are strongly influenced by the balance between the effects of pro-inflammatory and anti-inflammatory cytokines. Several cytokines have been suggested for their role in BPH (7). Cytokines are crucial regulators of cellular function and polymorphisms in cytokines related with BPH could be very important, not only for assessing the risk of BPH but also in helping to explain the pathogenesis and progression of disease.
IL10 is a multifunctional cytokine with anti-inflammatory and anti-angiogenic properties (12-14). IL10 has been reported to reduce tumor growth and angiogenesis (15-17). IL10 mediates tumor promoting and tumor suppressive activities such as apoptosis (18). A large number of polymorphisms have been identified in the IL10 gene promoter (19). Low IL10 producing genotypes were associated with increased susceptibility and advanced stage of several diseases such as malignant melanoma and renal cell carcinoma. However, high IL10 producing genotypes were associated with poor outcome of cervical cancer, gastric cancer, and hepatocellular carcinoma (19).
Several studies were reported in IL10 polymorphism related with prostate cancer and BPH (18, 20-24). Two of these studies found significant results. McCarron et al. (20) reported a significant association of the homozygous variant at -1082 with prostate cancer risk. Both the A/G and A/A genotype at -1082 have been associated with low IL10 production. The T allele at the -819 has been associated with low IL10 production. They concluded that SNP associated with differential production of IL10 are risk factor for prostate cancer acting via their influence on angiogenesis. Faupel-Badger et al. (24) examined the hypothesis that genotypes correlated with low IL10 production may be associated with increased prostate cancer risk among Finnish male participants from the Alpha-tocopherol Beta-carotene Cancer Prevention Study. The -819 T/T and -592 A/A low expression genotypes were highly correlated. These two genotypes also were associated with increased prostate cancer susceptibility and high grade tumors. These data revealed that genotypes correlated with low IL10 production are associated with increased risk of prostate cancer and with high-grade disease in Finnish male (24). Mullan et al. (18) reported the associations between BPH and polymorphism in genes that encode growth factor, cytokines, and vitamin D and receptor. The C/C genotype of the transforming growth factor-beta 1 gene was inversely associated with treatment for BPH. The presence of at least one allele with 17 or more CA repeats of the epidermal growth factor receptor gene was positively associated with high IPSS score. However, IL10 SNP (rs1800896) was not significantly associated with BPH.
In our study, G/G genotype of rs2834167 had a significant association with high PSA level. Moreover, the G allele of rs1518111 and the C allele of rs1554286 were found to be significantly associated with large prostate volume. We suggest that IL10 may be linked to reduce tumor growth and angiogenesis. He et al. (6) reported that the IL10 levels of expressed prostate secretion were higher in type II and type IIIa chronic prostatitis patients than in controls. They concluded that IL10 presumably play an important role in the process of prostate inflammation.
The limitation of our study was small sample number used for comparison within BPH group. But we have performed the first genetic examination of the relationships between IL10, IL10RA and IL10RB SNPs, and BPH, using standard diagnostic tools. Our results revealed a strong association between rs1518111, rs1554286, rs999788, and rs2834167 SNPs and BPH.
In conclusion, there is a strong association between the IL10 SNPs (rs1518111, rs1554286) and IL10RB SNPs (rs999788, rs2834167), and BPH in Korean population. Although our results have confirmed an association of IL10 and IL10RB SNP and BPH, additional investigation is needed for understanding protein expression in BPH.
Figures and Tables
 | Fig. 1Gene map and single nucleotide polymorphisms in the IL10 (A), IL10RA (B), and IL10RB (C) genes. Exons are marked with black boxes, and 5'-untranslated regions are marked with white boxes. The first nucleotide is denoted as + 1. Arrows indicate the location of each SNP. |
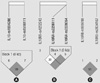 | Fig. 2Linkage disequilibrium (LD) blocks are consisted in IL10 (A) and IL10RA (B). LD block in IL10RB (C) is not consisted. IL10, interleukin-10; IL10RA, interleukin-10 receptor, alpha; IL10RB, interleukin-10 receptor, beta. |
Table 3
Analysis of genotype frequencies in IL10, IL10RA, and IL10RB gene polymorphisms, based on low (< 1.5 ng/mL) and high (≥ 1.5 ng/mL) PSA level, in subjects with BPH
AUTHOR SUMMARY
Association of IL10, IL10RA, and IL10RB Polymorphisms with Benign Prostate Hyperplasia in Korean Population
Koo Han Yoo, Su Kang Kim, Joo-Ho Chung, and Sung-Goo Chang
Inflammation has been documented in benign prostatic hyperplasia (BPH). Cytokines such as interleukin 10 (IL10) may play an important role in the process of inflammation. Therefore we analyzed the association between the BPH and single nucleotide polymorphisms (SNPs) of IL10, IL10RA and IL10RB in Korean population (233 BPH subjects and 214 age-matched healthy subjects). All patients with BPH were divided into two groups according to IPSS, PSA level, Qmax, and prostate volume. Genotypes of seven SNPs were determined through direct sequencing. The T/T genotype of rs999788 and the G/G genotype of rs2834167 were associated with a high PSA level. Of IL10 SNP, the A/A genotype of rs1518111 and the T/T genotype of rs1554286 were associated with small prostatic volume. Moreover, the T/T genotype of rs999788 was associated with high prostatic volume. We conclude that there is a strong association between the IL10 and IL10RB SNPs, and BPH in Korean population.
References
1. Park HK, Park H, Cho SY, Bae J, Jeong SJ, Hong SK, Yoon CY, Byun SS, Lee SE, Kim KW. The prevalence of benign prostatic hyperplasia in elderly men in Korea: a community-based study. Korean J Urol. 2009. 50:843–847.
2. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007. 51:1202–1216.
3. Konwar R, Gara R, Singh M, Singh V, Chattopadhyay N, Bid HK. Association of interleukin-4 and interleukin-1 receptor antagonist gene polymorphisms and risk of benign prostatic hyperplasia. Urology. 2008. 71:868–872.
4. Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988. 12:271–286.
5. Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002. 52:43–58.
6. He L, Wang Y, Long Z, Jiang C. Clinical significance of IL-2, IL-10, and TNF-alpha in prostatic secretion of patients with chronic prostatitis. Urology. 2010. 75:654–657.
7. Konwar R, Chattopadhyay N, Bid HK. Genetic polymorphism and pathogenesis of benign prostatic hyperplasia. BJU Int. 2008. 102:536–544.
8. Kaplan SA, McConnell JD, Roehrborn CG, Meehan AG, Lee MW, Noble WR, Kusek JW, Nyberg LM Jr. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 ml or greater. J Urol. 2006. 175:217–220.
9. Siami P, Roehrborn CG, Barkin J, Damiao R, Wyczolkowski M, Duggan A, Major-Walker K, Morrill BB. CombAT study group. Combination therapy with dutasteride and tamsulosin in men with moderate-to-severe benign prostatic hyperplasia and prostate enlargement: the CombAT (Combination of Avodart and Tamsulosin) trial rationale and study design. Contemp Clin Trials. 2007. 28:770–779.
10. Lewis CM. Genetic association studies: design, analysis and interpretation. Brief Bioinform. 2002. 3:146–153.
11. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005. 21:263–265.
12. Bagnoli S, Cellini E, Tedde A, Nacmias B, Piacentini S, Bessi V, Bracco L, Sorbi S. Association of IL10 promoter polymorphism in Italian Alzheimer's disease. Neurosci Lett. 2007. 418:262–265.
13. Deans DA, Tan BH, Ross JA, Rose-Zerilli M, Wigmore SJ, Howell WM, Grimble RF, Fearon KC. Cancer cachexia is associated with the IL10 -1082 gene promoter polymorphism in patients with gastroesophageal malignancy. Am J Clin Nutr. 2009. 89:1164–1172.
14. Heiskanen M, Kähönen M, Hurme M, Lehtimäki T, Mononen N, Juonala M, Hutri-Kähönen N, Viikari J, Raitakari O, Hulkkonen J. Polymorphism in the IL10 promoter region and early markers of atherosclerosis: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2010. 208:190–196.
15. Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. 1996. 2:1969–1979.
16. Kundu N, Beaty TL, Jackson MJ, Fulton AM. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. J Natl Cancer Inst. 1996. 88:536–541.
17. Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999. 5:189–196.
18. Mullan RJ, Bergstralh EJ, Farmer SA, Jacobson DJ, Hebbring SJ, Cunningham JM, Thibodeau SN, Lieber MM, Jacobsen SJ, Roberts RO. Growth factor, cytokine, and vitamin D receptor polymorphisms and risk of benign prostatic hyperplasia in a community-based cohort of men. Urology. 2006. 67:300–305.
19. Howell WM, Rose-Zerilli MJ. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Fam Cancer. 2006. 5:143–149.
20. McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA, Howell WM. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002. 62:3369–3372.
21. Eder T, Mayer R, Langsenlehner U, Renner W, Krippl P, Wascher TC, Pummer K, Kapp KS. Interleukin-10 [ATA] promoter haplotype and prostate cancer risk: a population-based study. Eur J Cancer. 2007. 43:472–475.
22. Xu J, Lowey J, Wiklund F, Sun J, Lindmark F, Hsu FC, Dimitrov L, Chang B, Turner AR, Liu W, Adami HO, Suh E, Moore JH, Zheng SL, Isaacs WB, Trent JM, Grönberg H. The interaction of four genes in the inflammation pathway significantly predicts prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005. 14(11 Pt 1):2563–2568.
23. Michaud DS, Daugherty SE, Berndt SI, Platz EA, Yeager M, Crawford ED, Hsing A, Huang WY, Hayes RB. Genetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancer. Cancer Res. 2006. 66:4525–4530.
24. Faupel-Badger JM, Kidd LC, Albanes D, Virtamo J, Woodson K, Tangrea JA. Association of IL-10 polymorphisms with prostate cancer risk and grade of disease. Cancer Causes Control. 2008. 19:119–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download