Abstract
Xanthogranulomatous inflammation (XGI) is a rare, idiopathic process in which lipid-laden histiocytes are deposited at various locations in the body. Although XGI has been reported to occur in various organs such as the gallbladder, kidney, bone, stomach, colon, appendix, lymph nodes, urachus, and urinary bladder and in soft tissues, xanthogranulomatous pancreatitis (XGP) is extremely rare. Herein, we report a case of XGP occurring in a 70-yr-old woman, who presented with abdominal pain for several months. On physical examination, mild epigastric tenderness was noted. Abdomen CT scan revealed a low attenuated mass in uncinate process of pancreas, suggesting malignant lesion. Whipple's operation was performed and the final pathologic diagnosis was XGP. The patient's post-operative course was uneventful, and no recurrence was found within 7 months of the operation. When a pancreatic mass does not show clinico-radiological features typical of common pancreatic neoplasms, XGP should be considered for a differential diagnosis.
Xanthogranulomatous inflammation (XGI) is a mixed inflammatory process characterized by infiltration of lipid-laden foamy histiocytes and other inflammatory cells along with marked fibrosis and parenchymal destruction. XGI occurs in various organs such as the skin, retroperitoneum, intracranium, gastrointestinal tract, genitalia, lymph nodes, urachus, and urinary bladder and in soft tissues (1-3). Xanthogranulomatous pancreatitis (XGP) is extremely rare, and currently only 12 cases of XGP have been reported in the English literature (4-13). In most cases, XGP was preoperatively misdiagnosed as pancreatic tumor. Herein, we report a case of XGP occurring in a 70-yr-old woman, who was initially misdiagnosed with pancreatic neoplasm, and later finally diagnosed with XGP.
A 70-yr-old Korean woman was admitted to our hospital on February 7, 2010 because she was experiencing abdominal pain and dyspepsia since the past 2 months. She had been treated for diabetes mellitus 12 yr ago and for hypertension 21 yr ago. She had had a cerebrovascular accident 2 yr ago. Her family history and the findings of a physical examination were unremarkable. The result of the laboratory test were as follows: serum levels of AST 28 (< 40 IU/L), ALT 29 (< 40 IU/L), total bilirubin 0.7 (0.2-1.3 mg/dL), amylase 51 (22-85 U/L), C-reactive protein (CRP) 3.0 (< 0.3 mg/dL), hemoglobin A1c 10.5 (< 6.0%); CEA 2.4 (< 6.0 ng/mL), CA 19-9 29.5 (< 27 U/mL), and CA 15-3 8.25 (< 30 U/mL). Computed tomography (CT) scan revealed a 2.2 cm sized, low-attenuated mass with peripheral enhancement around the uncinate process of the pancreatic head (Fig. 1A). Magnetic resonance imaging (MRI) demonstrated that the mass appeared hyperintense on a T2-weighted image and isointense on a T1-weighted image (Fig. 1B). Positron emission tomography-computed tomography (PET-CT) scan showed strong fluorodeoxyglucose (FDG) uptake in the mass at the pancreas uncinate process, suggesting a malignant condition (Fig. 1C). These findings suggested that the mass was a solid one rather than a cystic tumor. On the basis of the diagnosis of malignant neoplasm of the pancreas, laparotomy was performed on March 29, 2010. A slight, firm mass was seen around the uncinate process of the pancreas. Whipple's operation was performed. The patient's post-operative course was uneventful, and no recurrence was found within 4 months of the operation.
Gross examination of the resected specimen showed a 2.2 × 2.0 cm mass in the pancreas. The cut surface of the mass had a bright yellow color with irregular contour and thin fibrous septa (Fig. 2A). The adjacent pancreatic parenchyma was relatively intact. Microscopic examination from the mass revealed an aggregation of many foamy histiocytes, lymphocytes, and plasma cells. Low-power examination revealed dilated peripheral ducts, micropapillary proliferation in the small duct epithelium, and ductular proliferation (Fig. 2B). Focal parenchymal hemorrhage was also noted. The mixed inflammatory cells had infiltrated into the pancreatic parenchyma, displacing the normal acini, and extending into the peripancreatic adipose tissue. The acini were atrophied, and the residual islets were inflamed and surrounded by XGI (Fig. 2C). Immunohistochemical study showed a strong positive reactions for CD68 (Fig. 2D) and negative for desmin and Alk-1. These findings led to the diagnosis of XGP.
XGI is a rare form of chronic mixed inflammatory process characterized by lipid-laden histiocytes aggregation and mass formation. Such lesions have been described at many other sites, such as the kidney, urinary bladder, urachus, gallbladder, appendix, mandible, retroperitoneum, third ventricle, choroid plexus, orbit, vagina, lung, stomach, pericardium, and ovary (1-3). However, the occurence of XGI in the pancreas is extremely rare. To our knowledge, thus far, only 12 cases of XGP have been reported in the English literature (4-13). To the best of our knowledge, the present case is the 3rd case in Korea and the 14th case among the international cases. We have described here the clinico-pathological features of our case and compared it with previously reported cases of XGP.
According to previous reports, XGP occurs mainly in male patients (male to female ratio, 9:4), and the patients' mean age was 56 yr (22-82 yr). The most common symptom was abdominal pain (8/13 cases). The sites of the lesions were evenly distributed along the pancreatic parenchyma: head (4/13), body (5/13) and tail (4/13). Importantly, the preoperative diagnosis of 7 cases (7/13) was malignancy, including ductal adenocarcinoma (5, 8), intraductal papillary mucinous neoplasm (6, 9, 13) and solid pseudopapillary tumor (10). Six cases had associated inflammatory conditions including acute (4, 7) and chronic pancreatitis (11, 12). Prior to surgery, the patient in our case was also diagnosed with malignancy (Table 1). All the cases, including ours were treated with surgery; distal pancreatectomy (4, 7-9), Whipple's operation (5, 6), pylorus preserving pancreaticoduodenectomy (PPPD) (10-13), and mass excision (5). In addition to the 6 lesions, which were preoperatively diagnosed as neoplasms, 6 cases of inflammatory lesions were also surgically treated. Three acute inflammatory lesions needed surgical intervention because they progressed to pseudocysts and abcess (4, 7). Three other cases of chronic pancreatitis also were treated with PPPD to rule out malignancy (11, 12). After surgery, the overall postoperative courses of the patients were uneventful, and their amylase levels returned to normal (4, 7, 9).
The pathogenesis of XGP is not well understood. XGI is an uncommon inflammatory response. A relatively large number of cases of xanthogranulomatous cholecystitis and xanthogranulomatous pyelonephritis have been reported and several possible hypotheses of XGI have been proposed including defective lipid transport, hemorrhage, immunological disorders, reaction to a specific infectious agent of low virulence, and lymphatic obstruction (2). Xanthogranulomatous cholecystitis appears to result from the accumulation of histiocytes for the phagocytosis of extravasated bile in the gallbladder wall, which occur secondary to the rupture of Rokitansky-Aschoff sinuses and mucosal ulceration (2). Xanthogranulomatous pyelonephritis is considered to be caused by occlusion of the urinary tract and by infection (3).
Several hypotheses regarding the pathogenesis of XGP have been suggested. In cases with pancreatic duct dilatation or pseudocyst formation, it could be suspected that infection and hemorrhage occurred in a cyst formed by cystic neoplasm or after acute pancreatitis and elevated intracystic pressure, leading to xanthogranulomatous changes around the pancreatic cyst wall. Ueno et al. reported xanthogranulomatous changes in the wall of a pancreatic pseudocyst (4). They thought that these changes were the results of infection and hemorrhage in the pseudocyst following acute pancreatitis and elevated intracystic pressure. Kamitani and Kim et al. described XGP associated with an intraductal papillary mucinous tumor (IPMT); they speculated that mucin produced by the IPMT increased the intraductal pressure and that the subsequent leak of mucin into the pancreatic parenchyma produced the xanthogranulomatous changes (6, 13). Iyer et al. (5) reported 2 cases of XGP as mass lesions, suggesting that obstruction of pancreatic ducts by stones, followed by secondary bacterial infection, had initiated these responses.
Our case showed a solid mass in the uncinate process of the pancreatic head, however, cystic lesions (including pseudocyst and IPMT), duct stones and active inflammation (acute pancreatitis) were not found. However, dilation of the branched ducts branch and ductular proliferation were noted in XGP, which suggested local obstruction occurred. This ductal obstruction and the patient's 12 yr history of diabetes mellitus may have provoked a longstanding inflammatory milieu in the pancreatic parenchyma leading to XGP, which presented as a 2.2 × 2.0 cm solid mass. Our case suggests that XGP may occur by minor branch obstruction without stone, hemorrhage or acute inflammation.
Treatment of XGP may differ according to various events preceding XGP. In acute inflammatory lesions including pseudocyst and abscess, surgical intervention is treatment of choice irrespective of the presence of XGP. Surgical exploration is also considered to be compulsory when cystic neoplasms (e.g., IPMT) are considered as preoperative diagnosis. However, preoperative needle biopsy might be helpful, especially in cases a solid mass to avoid over-treatment.
In conclusion, as observed in the present case and in previous reports, most cases of XGP mimic primary pancreatic neoplasm. It is difficult to differentiate XGP from true neoplastic lesions on the basis of abdominal imaging studies. Although this type of pancreatitis is extremely rare, it is important for pathologists, radiologists, and clinicians to recognize this uncommon entity.
Figures and Tables
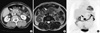 | Fig. 1Radiologic images of the pancreatic mass. (A) Abdominopelvic computed tomography scan revealed a low attenuated mass with peripheral enhancing and lobulating contour at the pancreatic uncinate process (arrow). (B) Pancreas magnetic resonance imaging demonstrated a mass having heterogenous hyperintense with hypointensity of its margin on a T2-weighted image (arrow). (C) Positron emission tomography for a differential diagnosis showed strong fluorodeoxyglucose uptake in mass of pancreas uncinate process about 3 cm in size. |
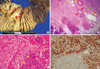 | Fig. 2Pathologic findings of the XGP. (A) Resected specimen shows an elliptical solid mass having bright yellow cut surface with fibrous septa (red arrows). (B) The xanthogranuloma extends into pancreatic parenchyma and peripancreatic adipose tissue. Dilatated peripheral ducts (yellow arrows), micropapillary proliferation of small duct (red arrows) and ductular proliferation (green arrows) are noted. (C) High power view reveals atrophy of acini and residual islets with inflammation (yellow arrows). (D) CD68 immunohistochemistry shows that strong positive xathogranuloma cells surround the acinus (red arrows). |
References
1. Kwak JE, Kim HS, Joo M, Chang SH, Shim SH, Chi JG, Cho IR. Combined xanthogranulomatous urachitis and bullous cystitis: a case report. Korean J Pathol. 2008. 42:41–44.
2. Houston JP, Collins MC, Cameron I, Reed MW, Parsons MA, Roberts KM. Xanthogranulomatous cholecystitis. Br J Surg. 1994. 81:1030–1032.
3. Choi YH, Choi WH, Han SY, Han KH, Kim HS. A case of bilateral xanthogranulomatous pyelonephritis with renal failure. Korean J Nephrol. 2008. 27:137–140.
4. Ueno T, Hamanaka Y, Nishihara K, Nishida M, Nishikawa M, Kawabata A, Yamamoto S, Tsurumi M, Suzuki T. Xanthogranulomatous change appearing in the pancreas cyst wall. Pancreas. 1993. 8:649–651.
5. Iyer VK, Aggarwal S, Mathur M. Xanthogranulomatous pancreatitis: mass lesion of the pancreas simulating pancreatic carcinoma--a report of two cases. Indian J Pathol Microbiol. 2004. 47:36–38.
6. Kamitani T, Nishimiya M, Takahashi N, Shida Y, Hasuo K, Koizuka H. Xanthogranulomatous pancreatitis associated with intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2005. 185:704–707.
7. Okabayashi T, Nishimori I, Kobayashi M, Sugimoto T, Kohsaki T, Okamoto K, Ito S, Moriki T, Araki K, Onishi S. Xanthogranulomatous pancreatic abscess secondary to acute pancreatitis: two case reports. Hepatogastroenterology. 2007. 54:1648–1651.
8. Shima Y, Saisaka Y, Furukita Y, Nishimura T, Horimi T, Nakamura T, Tanaka K, Shibuya Y, Ozaki K, Fukui Y, Hamada M, Nishioka Y, Okabayashi T, Taniki T, Morita S, Iwata J. Resected xanthogranulomatous pancreatitis. J Hepatobiliary Pancreat Surg. 2008. 15:240–242.
9. Iso Y, Tagaya N, Kita J, Sawada T, Kubota K. Xanthogranulomatous lesion of the pancreas mimicking pancreatic cancer. Med Sci Monit. 2008. 14:CS130–CS133.
10. Ikeura T, Takaoka M, Shimatani M, Koyabu M, Kusuda T, Suzuki R, Sumimoto K, Okazaki K. Xanthogranulomatous inflammation of the peripancreatic region mimicking pancreatic cystic neoplasm. Intern Med. 2009. 48:1881–1884.
11. Uguz A, Yakan S, Gurcu B, Yilmaz F, Ilter T, Coker A. Xanthogranulomatous pancreatitis treated by duodenum-preserving pancreatic head resection. Hepatobiliary Pancreat Dis Int. 2010. 9:216–218.
12. Kang BW, Kim JW, Jo JC, Lee SS, Seo DW, Lee SK, Kim MH. A case of xanthogranulomatous pancreatitis. Korean J Med. 2007. 72:Suppl 2. S171–S174.
13. Kim YN, Park SY, Kim YK, Moon WS. Xanthogranulomatous pancreatitis combined with intraductal papillary mucinous carcinoma in situ. J Korean Med Sci. 2010. 25:1814–1817.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download