Abstract
This study evaluated the structure of complex regional pain syndrome (CRPS) population and suggested a weighted scoring system to balance on objective signs. One hundred sixty-eight consecutive patients were evaluated using the Budapest Research Criteria (BRC). By using multidimensional scaling and logistic regression analysis, we analyzed the degree of importance and relationships between objective findings. In addition, a receiver operating characteristic curve was constructed using a weighted score derived from the risk ratio as a diagnostic test. There were correlations between skin color change and edema, and between decreased range of motion and motor dysfunction when multidimensional scaling was applied. The trophic change was excluded by a logistic regression (95% CI; 0.80-11.850). The cutoff point based on weighted score derived from the risk ratios for determining CRPS was 7.88. At this point, the sensitivity, specificity, positive predictive value and negative predictive value were 75.0%, 95.3%, 96.3%, and 70.1%, respectively. We propose a weighted scoring system for the BRC using risk ratios of objective signs. Although a thorough systematic review would be required in the future, this study can contribute to reduction of the possible distortion of the feature of CRPS populations by the BRC.
Since 1994, complex regional pain syndrome (CRPS) has been defined as a neuropathic pain condition caused by various circumstances, such as an extremity trauma and a peripheral nerve lesion (1). Although sequential stages of CRPS are refuted in patients who have received previous treatment (2), early detection is very important for the treatment (3). In spite of the importance of exact diagnosis for treatment, there is not a gold standard for the diagnosis of CRPS but several criteria (4). Therefore, the clinical approaches to patients who have CRPS remain difficult and largely empirical (5).
In the view of the modified diagnostic criteria of CRPS suggested by the Budapest group of International Association for the Study of Pain in 2004 (6), the diagnosis of CRPS is essentially clinical, which consists of 4 categories including sensory (category 1), vasomotor (category 2), sudomotor/edema (category 3), and motor/trophic (category 4). Clinical evaluation, however, is somewhat subjective with poor inter-observer correlation (7), which necessitates several complementary tests such as three-phase bone scan, simple radiographs, and/or digital infrared thermographic imaging even though their reliabilities remain controversial (8, 9).
In addition, for the Budapest diagnostic criteria for research (BRC), it recommended to adopting the combination of 4 symptoms from each category and at least 2 signs from different category as a research criteria, for which sensitivity was 70% and specificity was 94% (10). Its specificity, however, was rather decreased in spite of increasing the number of objective signs (11).
We hypothesized that the reason for that might be due to the number of subjective symptoms from distinct categories. According to the BRC (11), an extreme case that met 3 symptom categories and 4 sign categories, was classified as a case of non-CRPS. Signs such as sensory, decreased range of motion (ROM) or motor dysfunction could be fabricated intentionally even though motor signs are considerable to distinguish between CRPS and acute trauma (12), and sensory deficit and motor dysfunction are clinically prognostic factor of CRPS after stroke (13). Since the detailed information on the symptoms and signs of CRPS are easy to access via Internet or media by patients these days, aforementioned fabrication of symptoms may be increased, especially in a claim for compensation (14) and in an uneasy environment (15). Consequently, if the patients are selected by the BRC, the systematic error such as a publication bias may occur and then the features of CRPS tend to be deviated in the event.
For this reason, the purpose of this study was to evaluate the BRC through objective signs which contributed to the patient selection for representing the population of CRPS, and then to propose a new scoring system for the BRC.
The authors reviewed medical records of 168 consecutive patients who were evaluated using the BRC (11) at our university-based Pain Management Center from January, 2007 through June, 2009. Since the focus of the study was on CRPS, cases of CRPS type II in which a nerve lesion clearly accompanied the clinical manifestations also were included.
The patients who were referred from other hospitals after the confirmation of CRPS were excluded. The patients who had any devices in their body, such as for stabilization and for neuromodulation, and who had history of sympathectomy within one year or history of sympathetic blockade within one month were also excluded.
To satisfy the BRC, the patients should have reported abnormal continuing or spontaneous pain and more than one symptom in all 4 categories, and had at least 2 signs from different categories, which were identified during initial evaluation. This involved obtaining a patient history to assess subjective symptoms, as well as a physical examination conducted by physician to assess the objective signs.
For objective evaluation of temperature asymmetry, we applied a digital infrared thermography (IRIS® 5000, MEDICORE Inc., Seoul, Korea) to the region of interest and the contralateral part (12, 16), and considered as the positive sign when the difference in temperature between two parts was greater than 1℃. The objective signs were divided into 8 items; sensory (hyperalgesia and/or allodynia), temperature asymmetry, skin color change or asymmetry, edema, sweating asymmetry, decreased ROM, motor dysfunction (weakness, tremor and/or dystonia), and trophic changes (skin, hair, nail). Each item was scored on a dichotomous scale as '1' and '0' according to the presence.
Statistical analysis was performed using SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA). Demographic data were presented as mean ± SD (range), or frequencies and compared using t-tests, chi-square and Fisher's exact test, as appropriate.
A logistic regression analysis was performed to assess the relationship between CRPS (dependent variable) and items of objective sign (independent variables), calculated the adjusted odds ratio (OR) with 95% confidence intervals (CI). P < 0.05 was considered as the level of statistical significance.
A multidimensional scaling (MDS) was used to assess the geometric properties of items of CRPS. Using a set of computational procedures, the degree of (dis)similarity between two variables can be converted into the geometric distance between two points on the MDS map. Although a MDS is not probabilistic or deterministic, it can present a graphical display of the data structure, which is much easier to understand than a table of numbers. The S-Stress level < 0.2 or the R-squared index > 0.6, as a goodness-of-fit measure, was accepted statistically in terms of the data interpretability and information loss, and therefore with high positive correlations near each other, and variables with strong negative correlations far apart (17).
A receiver operating characteristic (ROC) curve was constructed using a weighted score derived from the risk ratio (18, 19) as a diagnostic test for confirming the BRC for CRPS. An ROC curve is a graph of true positive rates (sensitivity) versus false positive rates (1-specificity) that corresponds to each possible cut point for a diagnostic test (20). The data to generate an ROC curve and computations for statistical analysis were also made using the same program.
The demographic and clinical variables of 168 patients are detailed in Tables 1 and 3. According to the BRC for CRPS, 104 patients (63 males and 41 females) were classified as a CRPS group. Four patients in CRPS group were CRPS type II which was documented neurophysiologic abnormalities. Demographic and clinical characteristics of the two groups (CRPS group and non-CRPS group), stratified by the BRC were similar except the pain intensity measured by 10-cm visual analogue scale (VAS) and the absolute value of temperature difference at the regions of interest by the infrared thermographic images. The CRPS group had significantly higher scores of VAS (P = 0.01) and greater the absolute value of difference in temperature (P < 0.01) compared to those of the non-CRPS group. In non-CRPS group, 23 patients (35.9%) met the clinical diagnostic criteria for CRPS, and 13 patients (12.5%) had only 2 objective signs in CRPS group (Table 3).
Table 2 presents the incidence of signs of the two groups. Sensory, skin color change, edema and motor dysfunction were common signs whereas sweating and trophic changes were relatively not in CRPS group. In comparisons between the two groups, the CRPS group was significantly associated with objective items more frequent than the other (all P < 0.01).
From the point of view of objective items, skin color change from category 2 and edema from category 3 were relatively correlated (Pearson r = 0.544; P < 0.01), and decreased ROM and motor dysfunction both from category 4 were also correlated (Pearson r = 0.523; P < 0.01). Therefore, we decided to modify the two items (decreased ROM and motor dysfunction) in category 4 to a combined form as com-RM (union of the decreased ROM and the motor dysfunction). Since they came from same category, they would have been accompanied with more adverse statistical effects than the items of skin color change and edema.
We regressed CRPS status on all objective items including sensory, skin color change, temperature differences, edema, sweating change, com-RM, and trophic changes. In the multivariate model shown in Table 4, trophic changes was excluded from the significant predictors of CRPS model; the adjusted OR of a case being CRPS positive when a trophic change was positive was 3.08 (95% CI; 0.80-11.85). In addition, the adjusted OR from the regression exaggerated the risk of sensory item twice as much the crude OR from a 2 by 2 contingency table. Overall accuracy of this logistic model was 0.85 and the ratio of -2 log likelihood was 98.61.
To evaluate the relationship among variables, the geometric structure of the CRPS group, as determined on MDS mapping using the Euclidian distance, is shown in Fig. 1. Model fit statistics (S-Stress = 0.107; R-squared index = 0.923) supported a 2-dimensional solution. Partitioning the MDS space according to the first dimension (dimension 1) was in accordance with frequencies of the items. The second dimension (dimension 2) seemed to reflect the correlation between each items. The MDS plot showed close inter-relationship between sensory and com-RM, and between skin color change and edema.
In spite of the importance of trophic changes as an objective sign, it was excluded in our logistic model. Therefore we thought that a scoring system for diagnostic criteria was needed. Since CRPS had a high prevalence in present population, the risk ratios (RR) shown in Table 4, were used for a simple weighted score derived from following formula: Score = Σ (RR × objective item).
An ROC curve was constructed using the weighted score. The diagnostic performance of weighted score for determining CRPS is shown in Table 5. When the BRC was used as the referential standard, the area under the ROC curve was 0.931 at the cutoff score of 7.88 for being included the research population of CRPS. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 75.0%, 95.3%, 96.3%, and 70.1%, respectively (Table 5 and Fig. 2).
The demographics in the present study were not in agreement with the previous papers in regard to sex. The reported incidence and prevalence of CRPS were higher in female (4, 21-23). In this study, however, the female to male ratio in CRPS was 1:1.54, because military conscription system is practiced in Korea (n = 34) (24). Despite the demographic differences, the component ratio of objective items was not largely different from previous studies (4, 6, 21, 22).
Interestingly, there were 2 clusters of objective sign items according to quadrant spaces from MDS plot. Since sensory, skin color change, edema and com-RM are originated from distinct categories, we could analogize that it is easy to satisfy the condition of the BRC if a patient reports all subjective symptoms. Skin color change and edema were quite correlated in CRPS group but there were only 2 patients (1.9%) without other objective items. On the other hand, 5 patients (4.8%) had only sensory and com-RM as objective signs among 13 patients who had only 2 objective signs in CRPS group. Although they were small portion of CRPS group in this study, it might bring about an error to put emphasis on sensory, decreased ROM and motor dysfunction in the future.
Our scoring system according to the ROC curve showed a similar outcome with sensitivity of 75% and specificity of 95.3% as the referential standard from the BRC which has sensitivity of 70% and specificity of 94% (11, 25). In addition, for making a clinical diagnosis which requires at least one items in three of the four symptom categories and at least one items in two or more sign categories, the best cutoff score was 5.1. The area under the ROC curve value was 0.984 at the cutoff point and sensitivity, specificity, PPV, and NPV were 87.4%, 100%, 100%, and 71.9% respectively, and also showed an improved outcome than former result of them (6, 11).
To our knowledge, this is the first attempt to apply a weighted scoring system to the BRC for CRPS based on the objective signs. Our weighted scoring system had three comparable characteristics to the BRC. First, it contained all objective signs without classification of category. Also, even after excluding the self-reported subjective symptoms, it had similar sensitivity and specificity with the BRC. Lastly, it could prevent the characteristics of CRPS from inclining to special signs because at least three objective signs should be included according to our scoring system.
There are some limitations to be addressed in our study. First, this was a university hospital-based study therefore this might just reflect the severity of CRPS patients selected in a university hospital rather than from the general population. Even though patients were chosen consecutively, however, no differences in sex, age, and duration of disease between the groups were noted. A second limitation was that medical history and physical examination during the initial visit were performed by several investigators, allowing for the lack of internal validity. However, standard forms and double-checking system by another physician were adopted, which reduced the possibility of deviations in anamnesis and physical examination.
Of course, there could be conflicted between using the RR and using adjusted OR for the weighting score. As mentioned in results, however, it led that trophic changes, an important objective sign, was discarded during statistical evaluation if adjusted OR is used instead of the RR.
The first diagnostic criteria for CRPS in 1994 did not include motor dysfunction even though they were seen frequently in these conditions (1). To complement the former, modified form was proposed four distinct categories based on a principle components analysis (6) even though the latter has not been officially endorsed by the International Association of the Study of Pain yet. The BRC has been expected to be equally important in the context of sample selection for outcome studies due to its specificity (10), but a doubtful point is that the patients who are selected by the BRC, could represent the CRPS group clearly without considering correlation between signs from distinct categories as shown in the present study. Therefore it is necessary that the BRC should be more sophisticated to represent the characteristics of CRPS including all objective signs, although these objective signs could be fabricated by patients like factitious disorder (15).
In conclusion, we propose a weighted scoring system for the BRC of CRPS using risk ratios of objective items to reduce the possible distortion of the feature of CRPS population from the BRC. Even though the sensitivity and the specificity could not far surpass the BRC introduced as a referential standard and a thorough systematic review would be required to establish the external validity, this study can contribute to organize a homogeneous group of CRPS for research by means of correcting the correlations among objective signs and of decreasing a confounding effect from the possible invention of subjective symptoms by patients.
Figures and Tables
 | Fig. 1Two-dimensional representation of the relationship between seven objective items from the sample of 104 patients with CRPS. Multidimensional scaling analysis shows 2 tight links between edema and skin color, and between sensory and com-RM. The S-Stress is 0.107 and the R-squared index is 0.923 as a goodness-of-fit measure. Com-RM, combined form of decreased range of motion and motor dysfunction. |
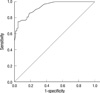 | Fig. 2Receiver operating characteristic (ROC) curve for diagnostic criteria of CRPS for research. The area under the ROC curve measures the performance of the test (0.931) and the best cutoff score was 7.88. The 45° dotted line represents the point at which the test is no better than chance. |
Table 4
Clinical properties of objective items for complex regional pain syndrome

Data are presented as odds ratio (OR) or risk ratios (RR) (95% confidence interval). Com-RM, combined form of decreased range of motion and motor dysfuction; ORcrude, crude OR, derived from 2 by 2 contingency table; ORadj, adjusted OR, derived from multivariate logistic regression analysis; RR, calculated according to following formula (Reference No. 18 and 19). RR = ORcrude / ([1-P0] + P0 × ORcrude) = ORcrude × (1-P1) / (1-P0). where P1 = positive predictive value and P0 = 1-negative predictive value. *P = 0.10.
AUTHOR SUMMARY
Proposing a Scoring System for the Research Criteria of Complex Regional Pain Syndrome
Kyoung Hoon Yim, Soo Young Park, Ji Yeon Yim, Yong Chul Kim, Sang Chul Lee, and Francis Sangun Nahm
We revisited the diagnostic criteria for Complex Regional Pain Syndrome (CRPS) to suggest a new weighted scoring system to balance the patients' objective signs. We evaluated 168 patients, and the analyzed results indicate correlations between skin color change and edema, and also between decreased range of motion and motor dysfunction. The trophic change was excluded by a logistic regression analysis. A receiver operating characteristic curve was constructed using a weighted score derived from the risk ratio as a diagnostic test. At a specific cutoff point, the sensitivity, specificity, positive predictive value and negative predictive value were 75.0%, 95.3%, 96.3%, and 70.1% respectively. We hope that the modified weighted scoring system might reduce the possible distortion of the CRPS populations by the previous criteria.
References
1. Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995. 63:127–133.
2. Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002. 95:119–124.
3. Park SG, Hyun JK, Lee SJ, Jeon JY. Quantitative evaluation of very acute stage of complex regional pain syndrome after stroke using three-phase bone scintigraphy. Nucl Med Commun. 2007. 28:766–770.
4. de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007. 129:12–20.
5. Dommerholt J. Complex regional pain syndrome-1: history, diagnostic criteria and etiology. J Bodyw Mov Ther. 2004. 8:167–177.
6. Harden RN, Bruehl S, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999. 83:211–219.
7. van de Vusse AC, Stomp-van den Berg SG, de Vet HC, Weber WE. Interobserver reliability of diagnosis in patients with complex regional pain syndrome. Eur J Pain. 2003. 7:259–265.
8. Schürmann M, Zaspel J, Löhr P, Wizgall I, Tutic M, Manthey N, Steinborn M, Gradl G. Imaging in early posttraumatic complex regional pain syndrome: a comparison of diagnostic methods. Clin J Pain. 2007. 23:449–457.
9. Pankaj A, Kotwal PP, Mittal R, Deepak KK, Bal CS. Diagnosis of post-traumatic complex regional pain syndrome of the hand: current role of sympathetic skin response and three-phase bone scintigraphy. J Orthop Surg (Hong Kong). 2006. 14:284–290.
10. Harden RN, Bruehl SP. Diagnosis of complex regional pain syndrome: signs, symptoms, and new empirically derived diagnostic criteria. Clin J Pain. 2006. 22:415–419.
11. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007. 8:326–331.
12. Birklein F, Künzel W, Sieweke N. Despite clinical similarities there are significant differences between acute limb trauma and complex regional pain syndrome I (CRPS I). Pain. 2001. 93:165–171.
13. Daviet JC, Preux PM, Salle JY, Lebreton F, Munoz M, Dudognon P, Pelissier J, Perrigot M. Clinical factors in the prognosis of complex regional pain syndrome type I after stroke: a prospective study. Am J Phys Med Rehabil. 2002. 81:34–39.
14. Mittenberg W, Patton C, Canyock EM, Condit DC. Base rates of malingering and symptom exaggeration. J Clin Exp Neuropsychol. 2002. 24:1094–1102.
15. Taskaynatan MA, Balaban B, Karlidere T, Ozgul A, Tan AK, Kalyon TA. Factitious disorders encountered in patients with the diagnosis of reflex sympathetic dystrophy. Clin Rheumatol. 2005. 24:521–526.
16. Kim YC, Bahk JH, Lee SC, Lee YW. Infrared thermographic imaging in the assessment of successful block on lumbar sympathetic ganglion. Yonsei Med J. 2003. 44:119–124.
17. Chang JS, Ahn YM, Yu HY, Park HJ, Lee KY, Kim SH, Kim YS. Exploring clinical characteristics of bipolar depression: internal structure of the bipolar depression rating scale. Aust N Z J Psychiatry. 2009. 43:830–837.
18. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998. 280:1690–1691.
19. Beaudeau F, Fourichon C. Estimating relative risk of disease from outputs of logistic regression when the disease is not rare. Prev Vet Med. 1998. 36:243–256.
20. Niehof SP, Beerthuizen A, Huygen FJPM, Zijlstra FJ. Using skin surface temperature to differentiate between complex regional pain syndrome type 1 patients after a fracture and control patients with various complaints after a fracture. Anesth Analg. 2008. 106:270–277.
21. Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009. 25:273–280.
22. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003. 103:199–207.
23. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999. 80:539–544.
24. Choi YS, Lee MG, Lee HM, Lee CJ, Jo JY, Jeon SY, Lee SC, Kim YC. Epidemiology of complex regional pain syndrome: a retrospective chart review of 150 Korean patients. J Korean Med Sci. 2008. 23:772–775.
25. Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK, Stanton-Hicks M. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. Pain. 1999. 81:147–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download