Abstract
The clinical significance of positive B-cell complement-dependent cytotoxicity crossmatching (B-CDC) in renal transplant recipients remains unclear. We reviewed 20 recipients with isolated B-CDC positivity at the time of transplantation. We compared the clinical characteristics, acute rejection and long-term graft survival between positive and negative B-CDC patients (n = 602). The number of retransplant recipients and positivity for T- and B-flowcytometric crossmatch was greater in positive B-CDC patients than in negative B-CDC patients. The overall acute rejection rate of positive B-CDC patients was significantly higher (P < 0.001), and Banff grade II or III cellular rejection was more frequently observed in positive B-CDC patients (P = 0.037). Compared with negative B-CDC patients, acute cellular rejection as a cause of graft loss was more prevalent (P = 0.020) and rescue rejection therapy was more frequently needed in positive B-CDC patients (P = 0.007). The allograft survival rate of positive B-CDC patients was significantly lower than that of negative B-CDC patients (P < 0.001), and B-CDC positivity independently increased the risk of allograft failure 2.31-fold (95% CI 1.15-4.67; P = 0.019) according to multivariate analysis. In conclusion, isolated B-CDC positivity is an independent long-term prognostic factor for allograft survival.
The complement-dependent cytotoxicity crossmatching test (CDC) is a widely used tissue-typing technique for detecting donor-specific anti-human leukocyte antigen (HLA) antibodies in renal transplantation. Because the CDC utilizes donor T- and B-cells, its interpretation is based on positivity for each cell type. Positive crossmatching on donor T-lymphocytes is recognized as the presence of anti-HLA class I antibodies and is a significant barrier to renal transplantation (1). In contrast, the value of a positive CDC for B lymphocytes (B-CDC) is unclear because B-cells do not express a uniform HLA antigen.
B lymphocytes express HLA class II molecules and constitutively express HLA class I molecules at a higher density than T-cells. Therefore, B-CDC positivity may reflect anti-HLA class II activity or weak anti-HLA class I activity. In addition, non-HLA antibodies and autoantibodies also result in positivity for B-CDC (2, 3). This heterogeneity of B-CDC contributes to the debates surrounding the effects of B-CDC positivity on renal transplantation with a concomitant negative T-CDC. Hyperacute vascular rejection in B-CDC positive recipients has been reported (4, 5), but some investigators have found that it has no influence on transplant outcome (6-12). Apart from these immediate anti-donor antibody responses, there are few reports indicating that B-CDC positivity influences long-term allograft survival or that renal transplant recipients with B-CDC positivity experience more adverse immunologic events.
Therefore, the aim of this study was to determine whether B-CDC positivity affects long-term renal allograft survival and whether positive B-CDC recipients experience more adverse immunologic events. We also sought to determine whether B-CDC positivity is independently predictive of renal allograft survival.
A retrospective review was performed of 735 renal transplant recipients from January 1995 to August 2008 at Seoul St. Mary's Hospital Transplantation Center. Of these patients, 113 cases were excluded because of T-CDC positivity (n = 4), unavailable flowcytometric crossmatching test (FCXM) results (n = 85) or incomplete medical records (n = 24). Therefore, 20 renal transplant recipients with a positive B-CDC result and 602 recipients with a negative B-CDC result were enrolled in this study. Clinical data including demographics, pretransplant immunologic status and biopsy-proven acute rejection were collected. Allograft loss was defined as return to maintenance dialysis, transplant nephrectomy or patient death with or without a functioning graft.
Donor T- and B-cells were isolated using CD19 monoclonal antibody attached to beads. One microliter of donor cell suspension (2 × 106 cells/mL) was incubated with 1 µL of recipient serum for 30 min at room temperature. Anti-human globulin 1 µL was added and incubated for 30 min at 37℃. After washing the cells, rabbit complement was added and incubated for 60 min at room temperature. The cells were stained with acridine orange and ethidium bromide, and observed for cytotoxicity using an immunofluorescent microscope. CDC results for T- and B-cells were considered positive when cell death exceeded that of the negative control well by 20%.
For the assay, 2 × 105 donor lymphocytes were added to 50 µL of patient serum and then incubated for 30 min at room temperature. Fluorescein isothiocyanate-labeled anti-human IgG (DAKO, Tokyo, Japan) and phycoerythrin-labeled CD19 or CD3 (DAKO) were added and incubated for 30 min. After washing the cells, a Coulter EPICS XL (Beckman Coulter, San Diego, CA, USA) was used for analysis. A positive FCXM was defined as a displacement of the mean channel fluorescence (MCF) by more than 10 channels relative to a negative control and donor autologous control. We also confirmed positive cases as having a relative median fluorescence (test MCF ÷ [recipient autologous MCF + donor autologous MCF + healthy autologous MCF] / 3) ≥ 1.5 and a test MCF greater than that of the negative MCF + 3SDs.
Panel-reactive antibody (PRA) tests and antibody monitoring system (AMS; GTI Inc., Waukesha, WI, USA) were used to detect the presence of donor-specific anti-HLA antibodies (DSA) in recipients. PRA was determined by enzyme-linked immunoabsorbent assays (ELISA) (LAT, One Lambda Inc., CA, USA). Positive sera were defined as those with values ≥ 20% of the positive control. When anti-HLA antibodies were positive by ELISA-PRA, the HLA specificity was confirmed by PRA identification. If the detected anti-HLA antibodies corresponded with relevant donor HLA antigen, we confirmed the detected anti-HLA antibodies as DSA. AMS is a solid-phase ELISA crossmatch test for detecting IgG antibody to the donor-specific solubilized HLA class I and class II antigens. We previously reported that AMS is useful as a supportive crossmatch test or as a monitoring test for detecting class I or II donor-specific anti-HLA antibodies (13, 14). The results of PRA screening were known in 233 (37.5%) patients (5 in positive B-CDC and 228 in negative B-CDC patients, and DSA detection was available in 226 (36.3%) patients (4 in positive B-CDC and 222 in negative B-CDC patients).
The patients received initial immunosuppression with a calcineurin inhibitor in combination with mycophenolate mofetil and prednisolone after transplantation. If recipients were highly sensitized, renal transplantations were performed after a desensitization protocol involving plasmapheresis and intravenous immunoglobulin (15). Cyclosporine was used for 442 patients, and the others were treated with tacrolimus. The initial dose of cyclosporine A was 10 mg/kg per day by the oral route; the target trough levels were 200-400 ng/mL during the first 4 weeks and 100-200 ng/mL thereafter. The initial dose of FK506 was 0.16 mg/kg per day by the oral route, and target trough levels were 8-15 ng/mL during the first 3 months and 3-8 ng/mL thereafter. Methylprednisolone (1 g/day) was administered by intravenous infusion on the day of transplantation, and the dose was then tapered to prednisone at 30 mg/day on the fourth day after transplantation. Mycophenolate mofetil (1.5 g/day) was initially used for 338 recipients, and the dose was modified to minimize adverse effects such as diarrhea or leukopenia.
Acute rejection was diagnosed by core needle biopsy. We reviewed the pathological evidence of acute rejection and graded it according to the Banff 97 working classification of renal allograft pathology (16). Hyperacute or accelerated rejection was considered as acute antibody-mediated rejection (AMR) based on pathological findings. Acute cellular rejection was treated with 3-4 daily boluses of intravenous methylprednisolone (500 mg/day), followed by a 5- to 7-day oral steroid taper. Anti-thymocyte globulin (ATG) or muromonab-CD3 (OKT3) was used as a rescue regimen when methylprednisolone was not effective against acute rejection.
The study was approved by the institutional review board of Seoul St. Mary's Hospital (KC10RISI0081). The board granted us an exemption for our retrospective study; additional patient informed consent was not required.
Descriptive statistical values are presented as the mean ± SD or median with range. Continuous variables with a normal distribution were analyzed using Student's t-test, and the chi-square test was used for analysis of categorical variables. Graft survival was analyzed using the Kaplan-Meier method with a log-rank test. A univariate test followed by a multivariate Cox-regression test was used to determine independent predictors of graft survival. Variables that showed a trend toward significance (P < 0.1) were included in the multivariate models. A P value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 16.0 software.
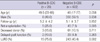
The age, sex and follow-up years were not different between positive and negative B-CDC patients (Table 1). There were many retransplant recipients and living unrelated donors in positive B-CDC patients. The incidence of positivity for T- (15.0% vs 3.8%) and B-FCXM (65.0% vs 8.8%) was higher in positive B-CDC patients than in negative B-CDC patients (Table 2). The percentage panel reactivity against HLA class I (%PRA I) and positivity for DSA class I were not significantly different. However, %PRA II values (65.0% vs 8.8%) and positive rates for DSA class II (50.0% vs 2.3%) were greater in positive B-CDC patients than in negative B-CDC patients. The pretransplant desensitization was more frequently performed (25.0% vs 2.5%) in positive B-CDC patients. Of these, three underwent desensitization because of a positive T-FCXM, one underwent desensitization because of high panel reactivity and one underwent desensitization because of retransplantation with a rejection history. In negative B-CDC patients, 15 patients underwent desensitization; the reasons were T-FCXM positivity (n = 13), high %PRA I and II (n = 1), and positive historic T-FCXM and current positive PRA I (n = 1).
Of the positive B-CDC patients, 15 episodes of acute rejection developed in 12 patients. The overall incidence of acute rejection was significantly higher in positive B-CDC patients than in negative B-CDC patients (14/20 vs 146/602; P < 0.001) (Table 3). Compared with negative B-CDC patients, peak serum creatinine level during rejection episodes was significantly higher in positive B-CDC patients (4.9 vs 3.3 mg/dL; P = 0.019). Acute rejections requiring ATG or OKT 3 rescue therapy were observed more frequently in positive B-CDC patients (35.7% vs 10.9%; P = 0.007). We compared rejection type between positive and negative B-CDC patients. No significant differences were observed in acute AMR (14.3% vs 4.0%; P = 0.138). However, grade II or III acute cellular rejection was significantly greater in positive B-CDC patients than in negative B-CDC patients (35.7% vs 14.5%; P = 0.037). The rate of grade I acute cellular rejection for positive B-CDC patients was lower than that for negative B-CDC patients (50.0% vs 81.5%; P = 0.005).
The allograft survival curve of positive B-CDC patients is illustrated in Fig. 1. The 5- and 10-yr graft survival rates were 70.0% and 43.8%, which were significantly lower than those of negative B-CDC patients (88.2% and 75.1%; P < 0.001). When patient death with a functioning graft was censored, positive B-CDC patients also showed a poorer graft survival rate than negative B-CDC patients (P = 0.005).
We compared the causes of allograft loss between positive and negative B-CDC patients (Table 4). The five patients (55.6%) with B-CDC positivity experienced graft loss caused by acute cellular rejection, the incidence of which was significantly greater compared with negative B-CDC patients (17.9%, P = 0.020). Acute AMR caused allograft loss in two (22.2%) positive B-CDC patients and in five (6.0%) negative B-CDC patients (P = 0.136). The incidence of chronic allograft nephropathy and patient death, as causes of graft loss were not significantly different between positive and negative B-CDC patients.
To identify prognostic factors associated with allograft survival, all of the clinical and immunologic parameters were analyzed by univariate and multivariate models (Table 5). Univariate analysis revealed that B-CDC positivity, HLA mismatch number and the number of acute rejection episodes were significant predictors of graft survival. The other variables that showed a trend toward significance (P < 0.1) were deceased donor and delayed graft function.
In the multivariate analysis, the independent predictors of allograft survival were B-CDC positivity, HLA mismatch number and number of acute rejection episodes. Deceased donor and delayed graft function were not significant in predicting graft survival. The relative risk was 2.31 for B-CDC positivity (95% confidence interval [CI], 1.15-4.67; P = 0.019), 1.21 for one HLA mismatch (95% CI, 1.04-1.41; P = 0.015) and 2.67 for one acute rejection episode (95% CI, 2.14-3.31; P < 0.001).
This study demonstrated that positive B-CDC patients showed a higher acute rejection rate and that Banff grade II or III rejection was more prevalent among positive B-CDC patients. Compared with negative B-CDC patients, rescue rejection therapy was needed more often for positive B-CDC patients, and acute cellular rejection resulted in graft loss more frequently in positive B-CDC patients. Furthermore, B-CDC positive patients had a lower allograft survival rate than negative B-CDC patients. These findings suggest that isolated B-CDC positivity is associated with poor renal allograft outcome.
Comparison of pretransplant immunologic status between positive and negative B-CDC patients revealed that positivity for T-FCXM was higher in positive B-CDC recipients (15.0% vs 3.8%). This finding suggests that B-CDC positivity increases the risk of acute AMR via the anti-HLA class I antibody (17, 18). Furthermore, 11 (55%) of the positive B-CDC patients were positive for B-FCXM but negative for T-FCXM, and the %PRA II value and DSA class II was higher in positive B-CDC patients. These findings suggest that B-CDC positivity is associated with sensitization for donor-specific anti-HLA class II antibody as well as anti-HLA class I antibody.
It is still controversial whether desensitization is needed in isolated positive B-CDC patients. In our center, desensitization is indicated in positive B-CDC recipients with high immunologic risk. However, of the patients who did not receive desensitization, two recipients experienced acute AMR, resulting in allograft loss. Interestingly, these patients were also positive for B-FCXM. This finding suggests that DSA against HLA class II may cause acute AMR, which is supported by previous reports that anti-HLA class II DSA is associated with the hyperacute rejection (19, 20). Therefore, pretransplant desensitization is suggested for patients with simultaneous positivity for B-CDC and B-FCXM.
Compared with negative B-CDC patients, peak serum creatinine level during rejection episodes and the prevalence of vascular rejection (Banff grades II or III) were significantly greater in positive B-CDC patients. Furthermore, acute rejections requiring rescue therapy and graft loss due to acute cellular rejection were more frequently observed in positive B-CDC patients. These findings suggest that acute rejections in B-CDC positive patients are more serious than those in negative B-CDC patients and that conventional anti-rejection treatment is not enough to overcome acute rejection in positive B-CDC patients. Otherwise, acute cellular rejection in positive B-CDC patients may be combined with antibody-mediated injury. Because humoral immunity is associated with vascular rejection, it may result in more adverse outcome of graft rejection in positive B-CDC patients (21-23).
Our study revealed that the incidence of acute AMR and allograft loss due to acute AMR were not significantly different between positive and negative B-CDC patients. However, the 5- and 10-yr graft survival rates of positive B-CDC patients were lower than those of negative B-CDC patients (70.0% vs 88.2% and 43.8% vs 75.1%). In the multivariate analysis, B-CDC positivity was as important as one acute rejection episode as an independent predictor of allograft survival (2.31 risk for B-CDC positivity vs 2.67 risk for one acute rejection episode). These findings suggest that B-CDC positivity contributes substantially to long-term kidney-transplant failure, although pretransplant desensitization in positive B-CDC patients can produce favourable short-term results.
The results of our study show the importance of B-CDC positivity in renal transplantation, but there are some limitations. First, the present study is observational, and immunosuppressive treatment was not consistent for each patient. Second, the PRA and AMS assay were restrictively allowed, because a solid-phase detection method was not available at the earlier time of study. Third, the lymphocytotoxic crossmatch test lacks specificity for HLA antigen and sensitivity for noncomplement fixing antibodies. Therefore, more sensitive ELISA or Luminex techniques for detecting DSA are needed considering a recent report showing that DSA is important for allograft survival in B-cell crossmatch-positive patients (24).
In conclusion, patients with B-CDC positivity are an immunologically high risk group for renal transplantation, and B-CDC positivity has an independent role in long-term renal allograft survival. Therefore, B-CDC positivity should be cautiously interpreted before renal transplantation.
Figures and Tables
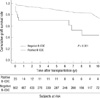 | Fig. 1Comparison of allograft survival between positive and negative B-CDC patients. Note that the survival rates for positive B-CDC patients was significantly lower than negative B-CDC patients (P < 0.001). |
Table 2
Pre-transplant immunologic status and immunosuppressants

Data expressed as means ± SD or number (percent). *The results of PRA screening were known in 233 (37.5%) of the 622 patients: 5 in the positive B-CDC patients and 228 in the negative B-CDC patients; †The results of DSA were known in 226 (36.3%) of the 622 patients: 4 in the positive B-CDC patients and 222 in the negative B-CDC patients. B-CDC, B-cell complement dependent cytotoxicity test; % PRA, percent panel reactive antibody; DSA, donor-specific antibody; FCXM, flowcytometric crossmatch test.
AUTHOR SUMMARY
B-cell Complement Dependent Cytotoxic Crossmatch Positivity is an Independent Risk Factor for Long-term Renal Allograft Survival
Hyeon Seok Hwang, Hye Eun Yoon, Bum Soon Choi, Eun Jee Oh, Ji Il Kim, In Sung Moon, Yong Soo Kim, and Chul Woo Yang
The clinical significance of positive B-cell complement-dependent cytotoxicity crossmatching (B-CDC) in renal transplant recipients remains unclear. We compared the clinical characteristics, acute rejection and long-term graft survival between positive and negative B-CDC patients. The positivity for T- and B-flowcytometric crossmatch was greater in positive B-CDC patients than in the negative. The incidence of acute rejection episodes was significantly greater in positive B-CDC patients. Also, the allograft survival rate of positive B-CDC patients was significantly lower than that of the negative. As a whole, B-CDC positivity increased the risk of allograft failure 2.31-fold.
References
1. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969. 280:735–739.
2. Girnita AL, Webber SA, Zeevi A. Anti-HLA alloantibodies in pediatric solid organ transplantation. Pediatr Transplant. 2006. 10:146–153.
3. Gebel HM, Bray RA, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Am J Transplant. 2003. 3:1488–1500.
4. Ahern AT, Artruc SB, DellaPelle P, Cosimi AB, Russell PS, Colvin RB, Fuller TC. Hyperacute rejection of HLA-AB-identical renal allografts associated with B lymphocyte and endothelial reactive antibodies. Transplantation. 1982. 33:103–106.
5. Scornik JC, LeFor WM, Cicciarelli JC, Brunson ME, Bogaard T, Howard RJ, Ackermann JR, Mendez R, Shires DL Jr, Pfaff WW. Hyperacute and acute kidney graft rejection due to antibodies against B cells. Transplantation. 1992. 54:61–64.
6. Ettinger RB, Terasaki PI, Opelz G, Malekzadeh M, Uittenbogaart C, Pennisi AJ, Fine R. Successful renal allografts across a positive cross-match for donor B-lymphocyte alloantigens. Lancet. 1976. 2:56–58.
7. Jeannet M, Benzonana G, Arni I. Donor-specific B and T lymphocyte antibodies and kidney graft survival. Transplantation. 1981. 31:160–163.
8. Ting A. Positive crossmatches --when is it safe to transplant? Transpl Int. 1989. 2:2–7.
9. Guerin C, Pomier G, Fleuru H, Laverne S, Le Petit JC, Berthoux FC. Renal transplantation with positive allocrossmatch (B/T; historical/current) but current T negative. Transplant Proc. 1991. 23:417–418.
10. Jendrisak M, Phelan D, Marsh J, McCullough C, So S, Mohanakumar T, Rush T, Michalski S, Hanto D. Significance of B-cell crossmatch on outcome in renal transplantation. Transplant Proc. 1991. 23:434–436.
11. Hourmant M, Bignon JD, Cesbron A, Soulillou J. Terasaki PI, editor. Effect of a positive crossmatch against donor B lymphocytes in cadaver kidney transplantation. A prospective one-center study. Clinical Transplants, UCLA Histocompatibility Typing. 1990. Los Angeles: Laboratory Press;301–309.
12. Ting A, Morris PJ. Renal transplantation and B-cell cross-matches with autoantibodies and alloantibodies. Lancet. 1977. 2:1095–1097.
13. Hwang HS, Hyoung BJ, Lee SY, Jeon YJ, Yoon HE, Kim JY, Choi BS, Oh EJ, Kim YS, Bang BK, Yang CW. Comparison of antibody monitoring system with flow cytometric crossmatch test in renal transplant recipients with high panel-reactive antibody. Nephron Clin Pract. 2009. 111:c260–c264.
14. Yang CW, Oh EJ, Lee SB, Moon IS, Kim DG, Choi BS, Park SC, Choi YJ, Park YJ, Han K. Detection of donor-specific anti-HLA class I and II antibodies using antibody monitoring system. Transplant Proc. 2006. 38:2803–2806.
15. Yoon HE, Hyoung BJ, Hwang HS, Lee SY, Jeon YJ, Song JC, Oh EJ, Park SC, Choi BS, Moon IS, Kim YS, Yang CW. Successful renal transplantation with desensitization in highly sensitized patients: a single center experience. J Korean Med Sci. 2009. 24:Suppl 1. S148–S155.
16. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999. 55:713–723.
17. Karpinski M, Rush D, Jeffery J, Exner M, Regele H, Dancea S, Pochinco D, Birk P, Nickerson P. Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol. 2001. 12:2807–2814.
18. Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, Cosio FG, Gandhi MJ, Kremers W, Stegall MD. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010. 10:582–589.
19. Feucht HE, Opelz G. The humoral immune response towards HLA class II determinants in renal transplantation. Kidney Int. 1996. 50:1464–1475.
20. Le Bas-Bernardet S, Hourmant M, Valentin N, Paitier C, Giral-Classe M, Curry S, Follea G, Soulillou JP, Bignon JD. Identification of the antibodies involved in B-cell crossmatch positivity in renal transplantation. Transplantation. 2003. 75:477–482.
21. Issa N, Cosio FG, Gloor JM, Sethi S, Dean PG, Moore SB, DeGoey S, Stegall MD. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008. 86:681–685.
22. Kooijmans-Coutinho MF, Hermans J, Schrama E, Ringers J, Daha MR, Bruijn JA, van der Woude FJ. Interstitial rejection, vascular rejection, and diffuse thrombosis of renal allografts. Predisposing factors, histology, immunohistochemistry, and relation to outcome. Transplantation. 1996. 61:1338–1344.
23. Salmela KT, von Willebrand EO, Kyllönen LE, Eklund BH, Höckerstedt KA, Isoniemi HM, Krogerus L, Taskinen E, Ahonen PJ. Acute vascular rejection in renal transplantation--diagnosis and outcome. Transplantation. 1992. 54:858–862.
24. Eng HS, Bennett G, Tsiopelas E, Lake M, Humphreys I, Chang SH, Coates PT, Russ GR. Anti-HLA donor-specific antibodies detected in positive B-cell crossmatches by Luminex predict late graft loss. Am J Transplant. 2008. 8:2335–2342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download