Abstract
To investigate the effects of reactive oxygen species (ROS) on tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) plasma levels, and their possible implications on clinical outcome, we measured tPA and PAI-1 levels in 101 patients with acute paraquat (PQ) intoxication. The control group consisted of patients who ingested non-PQ pesticides during the same period. tPA and PAI-1 levels were higher in the PQ group than in the controls. PQ levels were significantly correlated with ingested amount, timelag to hospital, tPA level, and hospitalization duration. tPA levels were correlated with PAI-1, fibrin degradation product (FDP), and D-dimer. D-dimer levels were lower in the PQ group than in the controls. Univariate analysis indicated the following significant determinants of death: age, ingested amount, PQ level, timelag to hospital, serum creatinine, lipase, pH, pCO2, HCO3-, WBC, FDP, PAI-1, and tPA. However, multivariate analysis indicated that only PQ level was significant independent factor predicting death. In conclusion, tPA and PAI-1 levels were higher, while D-dimer levels were lower in the PQ group than in the controls, implying that ROS stimulate tPA and PAI-1, but PAI-1 activity overrides tPA activity in this setting. Decreased fibrinolytic activity appears to be one of the clinical characteristics of acute PQ intoxication.
Tissue plasminogen activator (tPA) is a serine protease expressed by endothelial cells of blood vessels that catalyzes the conversion of plasminogen to plasmin (1). In addition to its role in the fibrinolysis system, tPA is implicated in tissue proliferation, cellular adhesion, and the activation of growth factors and matrix metalloproteinases (2). Insight into the regulation of the synthesis and release of tPA has extended markedly over the last 2 decades, including the characterization of the intracellular signaling pathways, promoter elements, and transcription factors involved in the transcriptional regulation of tPA (3, 4).
The tPA gene is located on chromosome 8 (5). Evidence from experimental studies indicates that reactive oxygen species (ROS) can inhibit tPA mRNA expression and synthesis, and impair the release of tPA from cultured human endothelium (6, 7). Furthermore, oxidants may alter the structural characteristics of tPA, which in turn may adversely affect endothelial fibrinolytic activity (8). However, these observations were derived from in vitro experiments or from experimental animal models. Van Guilder et al. observed that the antioxidant vitamin C increased the capacity of the endothelium to release tPA in overweight/obese adults (9). They concluded that ROS inhibit tPA production; however, it is not clear whether high levels of ROS suppress plasma tPA levels, leading to thrombosis in humans.
Plasminogen activator inhibitor-1 (PAI-1) is the principal inhibitor of tPA (10). It is mainly produced by the cells lining blood vessels, but it is also secreted by other tissue types, e.g., adipose tissue. The PAI-1 gene is located on chromosome 7 (7q21.3-q22) (11). There is little information regarding the effects of ROS on the production of PAI-1 (3, 12).
Paraquat (PQ, 1,1'-dimethyl-4,4'-bipyridinium dichloride) is one of the most commonly used herbicides in the world (13-15). In human, intentional or accidental ingestion of PQ is considered to be uniformly fatal, resulting in death from multi-organ failure and cardiogenic shock within few days (16). After ingestion of small quantities, PQ is specially taken up into and accumulates in the lung. Subsequent redox cycling and free radical generation triggers a neutrophil-mediated inflammatory response in the lungs, which initiates an irreversible fibrotic process that kills the majority of patients within several weeks (17). Considering that PQ is the most frequently used chemical to produce ROS in vitro experiments, acute PQ intoxication could be a human model to study specific clinical conditions related to ROS.
The purpose of this study was to examine the plasma levels of tPA and PAI-1 in patients with acute PQ intoxication, focusing on the relationship between these parameters and the clinical features of PQ intoxication.
Soonchunhyang Cheonan Hospital's Investigational Review Board approved this study, and all participants gave written informed consent. We enrolled 101 patients (58 males and 43 females, aged 49.1 ± 16.0 yr) with acute PQ intoxication in this study. All of the patients ingested concentrated PQ (22%-23% per volume) while committing suicide and were admitted to the Institute of Pesticide Poisoning, Soonchunhyang University Cheonan Hospital, from September 2009 to March 2010. The amount ingested was estimated from the number of swallows, where 1 mouthful was considered as 20 mL.
For the control group, we enrolled 30 patients (14 males and 16 females, aged 53.5 ± 16.6 yr) with acute non-PQ pesticide intoxication. The control group also ingested pesticides while committing suicide and were admitted to the same hospital during the same period. The frequency of ingested pesticides in the control group was as follows: organophosphate, 9 (30%); glyphosate, 6 (20%); glufosinate, 5 (16.7%); pyrethroid, 3 (10%); and others, 7 (23.3%). The demographic and basal laboratory findings for both groups of patients are summarized in Table 1.
Upon admission, the patients received the following standardized medical emergency procedures. Briefly, a gastric lavage was performed on all subjects who presented to the emergency room (ER) within 2 hr of PQ ingestion. For those whose intoxication occurred within 12 hr before presenting to the ER, 100 g of Fuller's earth in 200 mL of 20% mannitol was administered. Hemoperfusion was initiated early and continued for 4-6 hr according to the result of the urine dithionite test. Glutathione (50 mg/kg/24 hr) was administered intravenously for 3 additional days as an ROS scavenger. For the control group, early hemoperfusion was performed on the first day of hospitalization in the same manner as the PQ group and conservative therapy was followed. For patients with organophosphate intoxication, atropine and pralidoxime (PAM) were administered.
Blood samples were collected in the ER for baseline laboratory findings. The PQ levels were measured by the HPLC method.
There are various ways to estimate the tPA mediated fibrinolytic activity, such as measurement of tPA with catalytic activity and/or quantification of tPA antigen, as a free form or complex form of tPA-PAI-1. In this study, we measured tPA and PAI-1 antigen to observe the effect of ROS on the production and/or release of tPA and PAI-1 each. Of course, tPA and PAI-1 antigen are not consistent with the activity of tPA and PAI-1. In order to compensate for the defect, we measure fibrin degradation product (FDP) and D-dimer, which are always the end-products of fibrinolysis. Besides, they represent the pre-existence of thrombus.
To quantify tPA and PAI-1 levels, plasma samples were collected 3 times: the first sample was collected in the ER and the other 2 samples were taken on consecutive days at 24 hr intervals. tPA (Asserachrom® tPA kit; Diagnostica Stago, Asnières-Sur-Seine, France) and PAI-1 (Asserachrom® PAI-1 kit; Diagnostica Stago) antigen levels were measured using commercially available assay kits. tPA and PAI-1 levels were presented as the mean of these 3 measurements.
Continuous variables are presented as the mean ± standard deviation and categorical variables as frequency (in percent). The differences between the PQ and control groups were compared using Student's t-test for continuous variables and by the chisquared-test or Fisher's exact test for categorical variables. The relationship between variables was analyzed using bivariate correlation analysis. Univariate binary logistic regression analysis was used to verify the clinical implications of the fibrinolytic markers, e.g., tPA and PAI-1. Multivariate logistic regression analysis was used to identify significant determinants of death after PQ intoxication. The results of the logistic regression analyses were reported as odds ratio with 95% confidence intervals (CI). Statistical analyses were performed using SPSS software (version 14.0; SPSS Inc., Chicago, IL, USA). All P values below 0.05 were considered statistically significant.
tPA (24.8 ± 15.2 vs 18.0 ± 12.7 ng/mL, respectively, P = 0.038) and PAI-1 (69.9 ± 25.0 vs 51.3 ± 23.0 ng/mL, respectively, P = 0.001) levels were significantly higher in the PQ group than in the control group (Fig. 1). D-dimer levels were significantly lower in the PQ group than in the control group (1.2 ± 1.1 vs 2.1 ± 1.1 µg/mL, respectively, P = 0.001) (Fig. 2). However, there were no significant differences in the levels of FDP between the control and PQ groups (7.4 ± 3.3 vs 6.3 ± 3.9 µg/mL, respectively, P = 0.117).
Patients with PQ intoxication were divided into 2 subgroups according to their PQ level: ≥ 10 µg/mL vs < 10 µg/mL. Patients whose PQ levels were over 10 µg/mL had significantly higher levels of tPA than those below 10 µg/mL (36.3 ± 13.0 vs 18.3 ± 11.7 ng/mL, respectively, P < 0.001) (Fig. 3). In addition, bivariate correlation analysis revealed a significant correlation between PQ levels and the amount of PQ ingested (r = 0.281, P = 0.005), the time interval between PQ ingestion and hospital admission (r = -0.286, P = 0.004), the level of tPA (r = 0.417, P = 0.001) and hospitalization duration (r = -0.419, P < 0.001).
The amount of PQ ingested demonstrated a positive relationship with the levels of tPA (r = 0.579, P < 0.001) and PAI-1 (r = 0.428, P < 0.001) (Fig. 4); similarly, the levels of tPA were significantly correlated with the levels of PAI-1 (r = 0.621, P < 0.001), FDP (r = 0.479, P < 0.001), and D-dimer (r = 0.312, P = 0.014).
Fifty-six (55.4%) patients died in the PQ group; while 2 (6.7%) patients died in the control group. The demographic and laboratory findings for the survivors and non-survivors are summarized in Table 2. Briefly, the deceased patients were older, ingested larger amounts of PQ, arrived at hospital later, and had higher levels of PQ, tPA, and PAI-1 than those who survived.
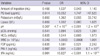
Univariate binary logistic regression analysis of the PQ group indicated that the following factors were significant determinants of death: age, amount of PQ ingested, PQ levels, time lag between PQ ingestion and admission, hospitalization duration, serum creatinine, potassium, lipase, pH, pCO2, HCO3-, white blood cell count (WBC), FDP, PAI-1, and tPA levels (Table 3). However, multivariate binary logistic regression analysis indicated that only PQ levels, from these factors, were a significant independent factor predicting death (Table 4).
Coagulation and fibrinolysis proceed concomitantly, since a thrombus is formed on a damaged vessel wall and leads to the release of tPA from the intact endothelium nearby the thrombus (18). Consequently, tPA activates the conversion of plasminogen to plasmin, inducing the so-called "thrombus-specific fibrinolysis (19)". Venous thrombosis has many different etiologies and occurs when several risk factors are present simultaneously (20). Risk factors in patients with acute pesticide intoxication, in addition to the traditional ones, include prolonged bed rest (21), placement of central venous catheters (22), and hemoperfusion (23). This is the reason why we recruited a control group consisting of patients with non-PQ pesticide intoxication who underwent similar treatment modalities as the PQ group, including bed rest, a central venous catheter for the extracorporeal elimination of poisons, and/or the intravenous administration of a large volume of fluid.
Contrary to the previous reports (6, 7, 9), we observed significantly higher levels of tPA and PAI-1 in the PQ group than in the control group (Fig. 1). Furthermore, the levels of tPA and PAI-1 were significantly higher in patients whose PQ levels were higher than 10 µg/mL (Fig. 3). We cannot explain this discrepancy, but in the previous reports (6, 7), the results were described from not in vivo but in vitro experiments. In another previous study (9), they reported decreased levels of tPA after the injection of vitamin C in human subjects. With this result, they concluded that ROS may inhibit tPA production. However, their theory is open to criticism because the function of vitamin C is complicated in ROS formation, not scavenging ROS but stimulate ROS production in some situation.
In our results, there was a significant correlation between the levels of tPA and/or PAI-1 with the amount of PQ ingested (Fig. 4). The plasma PQ level at a given time is a function of the amount ingested and the time lag after ingestion; therefore, the PQ level does not represent the severity of intoxication in our study because the patients arrived at the ER with different time lags after ingestion. However, it has been our clinical observation that no patients survive with a plasma PQ level > 10 µg/mL during the admission period. This observation led us to divide the PQ group according to their PQ levels: greater than and lower than 10 µg/mL.
This raises the question of whether these relationships are due to PQ itself or an ROS effect generated by PQ. ROS are formed primarily in cells; however, direct measurement of ROS formation in cells is very difficult in the clinical setting. Instead, several indirect methods have been developed to measure free radicals and their metabolites, e.g., malondialdehyde (MDA; the final product of lipid peroxidation) (24) and hydroperoxides (ROOH; intermediate products of lipid peroxidation) (25). Recently, we reported that neither cross-sectional nor sequential measurements of plasma MDA provided reliable data on ROS formation in patients with acute PQ intoxication (24). Given the lack of a reliable marker for ROS production, it is difficult to find a relationship between ROS levels and other parameters in acute PQ intoxication.
The plasma levels of PQ reach a peak level at 1.5 hr after PQ ingestion and decrease so rapidly that its levels are undetectable after 24 hr in the majority of patients; therefore, plasma PQ levels observed in the ER are not comparable with those from the subsequent days. Contrary to the PQ levels, the 3 measurements of tPA and PAI-1 levels taken in this study were comparable in each patient; the mean coefficient of variation was 0.42 ± 0.22 for tPA (range 0.08-1.02) and 0.39 ± 0.30 for PAI-1 (range 0.00-1.31). These results suggest that it is not the mere level of PQ that affects tPA and PAI-1 levels, but some other factor(s) activated or stimulated by PQ; therefore, we consider that ROS generated by PQ trigger the endothelium to release tPA and PAI-1.
D-dimer levels were lower in the PQ group than in the control group (Fig. 2). The presence of D-dimer indicates the pre-existence of fibrin formation and the concurrent formation of plasmin by tPA (26). Coagulation and fibrinolysis are on-going physiological processes that account for the presence of D-dimer. Fibrin formation is the result of the imbalance between coagulation and anticoagulation activities in a pathological environment. Therefore, the possibility exists that some thrombogenic factor such as tissue factor could have been activated during PQ intoxication; however, changes in coagulation/anticoagulation activity were not the focus of the current study. In the PQ group, tPA and PAI-1 levels were higher, but D-dimer levels were lower than in the control group, indicating that, in this setting, PAI-1 activity overrode tPA activity.
Taken together, these results indicate that thrombosis should be a common complication in patients with acute PQ intoxication, although only 2 of the 101 PQ patients suffered from pulmonary artery thrombosis or deep vein thrombosis (Figs. 5, 6). However, bearing in mind that venous thrombosis is often clinically silent, we believe that there must have been more patients with subclinical thrombosis complications in the PQ group.
In order to verify the clinical implications of the tPA and PAI-1 levels, we performed univariate and multivariate binary logistic regression analyses. Univariate binary logistic regression analysis of the PQ group showed that age, amount of PQ ingested, plasma PQ levels, time lag after PQ ingestion, tPA levels, and PAI-1 levels were significant determinants of death (Table 3). However, multivariate binary logistic regression analysis indicated that only PQ levels were a significant independent factor predicting death (Table 4).
We have few limitations in this study. First, we had better estimate the tPA mediated fibrinolytic activity with both tPA antigen and tPA activity. Furthermore, parameters of coagulation, such as a tissue factor and its inhibitors would have enhanced the significance in the interpretation of the change in both tPA and PAI-1 levels. However, in practice, it was hard to obtain blood samples frequently in large amount because of their critical condition. The other question posed is the lack of quantitative measurement of ROS.
As we described in the introduction, it was impossible to measure ROS directly and only surrogate markers were available in clinical practice. Even with these limit, our observations imply that ROS stimulate the production of tPA and PAI-1, but PAI-1 activity overrides tPA activity in this setting. Decreased fibrinolytic activity due to increased PAI-1 activity appears to be one of the clinical characteristics of acute PQ intoxication.
In conclusion, the levels of tPA and PAI-1 were higher, but D-dimer levels were lower in the PQ group than in the control group.
Figures and Tables
Fig. 1
Comparison of the tPA and PAI-1 levels between the control and PQ intoxication groups. Note that tPA and PAI-1 levels are significantly higher in the PQ group than in the control group.

Fig. 2
Comparison of the levels of D-dimer and fibvin degradation product (FDP) between the control and PQ intoxication groups. Note that the D-dimer levels are significantly lower in the PQ group than in the control group.

Fig. 3
Relationship between PQ levels and the levels of tPA and PAI-1 in PQ-intoxicated patients. PQ-intoxicated patients were divided into 2 subgroups according to their PQ level: ≥ 10 µg/mL vs < 10 µg/mL.

Fig. 4
Relationship between the amount of PQ ingested and the levels of tPA (A) and PAI-1 (B) in PQ-intoxicated patients. Note that there is a positive correlation between the amount of PQ ingested and the levels of tPA and PAI-1.

Fig. 5
Pulmonary artery computerized tomography angiogram from a PQ-intoxicated patient who suffered pulmonary artery thrombosis. A 64-yr-old woman swallowed a mouthful of a 24.5% PQ solution. Her plasma PQ level was 0.08 µg/mL at 15 hr after PQ ingestion. On day 9 of admission, the patient complained of right sided chest pain and dyspnea. Arterial blood gas analysis indicated: pH, 7.459; PaO2, 46.3 mmHg; and PaCO2, 37.9 mmHg. D-dimer was elevated at 2.9 (0-0.5) µg/mL. A chest X-ray showed an increased density along the apex of the right lung. (A) obtained at 9 days after PQ ingestion. Note the thrombus in the pulmonary artery and interlobar pulmonary artery (arrow a, b). A subcutaneous injection of low molecular weight heparin was started on the 9th day and warfarin was started on the 10th day. (B) obtained at 20 days after PQ ingestion. The thrombus was completely resolved (arrow c, d). The patient made a full recovery from this condition.
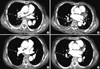
Fig. 6
Low extremity venogram computerized tomography from a PQ-intoxicated patient who suffered deep vein thrombosis in the leg. A 50-yr-old woman swallowed 2 mouthfuls of a 24.5% PQ solution. (A) On day 21 after admission, the patient complained of left leg swelling and pain, and a deep vein thrombosis was suspected. D-dimer was elevated at 25.6 µg/mL. (B) Deep vein thrombosis was noted in the left deep venous system (arrow a, b). (C, D) Obtained at 63-days after PQ ingestion. The extent of the thrombus was significantly reduced (arrow c, d).
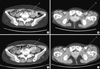
Table 2
Demographic and laboratory findings between the survived and deceased groups of paraquat-intoxicated patients

Table 3
Univariate binary logistic regression analysis to identify significant determinants of death in paraquat-intoxicated patients
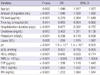
Table 4
Multivariate binary logistic regression analysis to verify significant determinants of death in paraquat-intoxicated patients. Values of tPA and PAI-1 were adjusted by age, sex, time interval between PQ ingestion and hospital arrival, PQ levels, amount of PQ ingested and serum creatinine levels
AUTHOR SUMMARY
Tissue Plasminogen Activator and Plasminogen Activator Inhibitor-1 Levels in Patients with Acute Paraquat Intoxication
Su-Jin Seok, Su-Ji Kim, Hyo-Wook Gil, Jong-Oh Yang, Eun-Young Lee, and Sae-Yong Hong
Paraquat (PQ), a toxic herbicide, is highly reactive to oxygen. Here, in PQ-intoxicated patients, we investigated the plasma levels of both tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1), and their possible implications on clinical outcome. We observed that the levels of both tPA and PAI-1 were higher, while D-dimer levels were significantly lower in the patients with PQ-intoxication than in the control group. Univariate analysis indicated the following significant determinants of death: age, ingested amount, PQ level, time lag to hospital, serum creatinine, lipase, pH, pCO2, HCO3-, WBC, FDP, PAI-1, and tPA. However, multivariate analysis indicated that only PQ level was significant independent factor predicting death. Our findings imply that PQ-induced ROS might stimulate tPA and PAI-1, but PAI-1 activity overrides tPA activity in this setting. Decreased fibrinolytic activity appears to be one of the clinical characteristics of acute PQ intoxication.
References
1. Collen D, Lijnen HR. Tissue-type plasminogen activator: a historical perspective and personal account. J Thromb Haemost. 2004. 2:541–546.
2. Zorio E, Gilabert-Estellés J, España F, Ramón LA, Cosín R, Estellés A. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. 2008. 15:923–929.
3. Nagamine Y. Transcriptional regulation of the plasminogen activator inhibitor type 1 with an emphasis on negative regulation. Thromb Haemost. 2008. 100:1007–1013.
4. Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006. 25:695–705.
5. Nusbaum C, Mikkelsen TS, Zody MC, Asakawa S, Taudien S, Garber M, Kodira CD, Schueler MG, Shimizu A, Whittaker CA, Chang JL, Cuomo CA, Dewar K, FitzGerald MG, Yang X, Allen NR, Anderson S, Asakawa T, Blechschmidt K, Bloom T, Borowsky ML, Butler J, Cook A, Corum B, DeArellano K, DeCaprio D, Dooley KT, Dorris L 3rd, Engels R, Glöckner G, Hafez N, Hagopian DS, Hall JL, Ishikawa SK, Jaffe DB, Kamat A, Kudoh J, Lehmann R, Lokitsang T, Macdonald P, Major JE, Matthews CD, Mauceli E, Menzel U, Mihalev AH, Minoshima S, Murayama Y, Naylor JW, Nicol R, Nguyen C, O'Leary SB, O'Neill K, Parker SC, Polley A, Raymond CK, Reichwald K, Rodriguez J, Sasaki T, Schilhabel M, Siddiqui R, Smith CL, Sneddon TP, Talamas JA, Tenzin P, Topham K, Venkataraman V, Wen G, Yamazaki S, Young SK, Zeng Q, Zimmer AR, Rosenthal A, Birren BW, Platzer M, Shimizu N, Lander ES. DNA sequence and analysis of human chromosome 8. Nature. 2006. 439:331–335.
6. Shatos MA, Doherty JM, Stump DC, Thompson EA, Collen D. Oxygen radicals generated during anoxia followed by reoxygenation reduce the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in human endothelial cell culture. J Biol Chem. 1990. 265:20443–20448.
7. Eberhardt W, Beck KF, Pfeilschifter J. Cytokine-induced expression of tPA is differentially modulated by NO and ROS in rat mesangial cells. Kidney Int. 2002. 61:20–30.
8. Feng YH, Hart G. In vitro oxidative damage to tissue-type plasminogen activator: a selective modification of the biological functions. Cardiovasc Res. 1995. 30:255–261.
9. Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. J Physiol. 2008. 586:3525–3535.
10. Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005. 93:631–640.
11. Asselbergs FW, Pattin K, Snieder H, Hillege HL, van Gilst WH, Moore JH. Genetic architecture of tissue-type plasminogen activator and plasminogen activator inhibitor-1. Semin Thromb Hemost. 2008. 34:562–568.
12. Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008. 100:969–975.
13. Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY, Hong SY. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med. 2009. 24:247–251.
14. Lee KH, Gil HW, Kim YT, Yang JO, Lee EY, Hong SY. Marked recovery from paraquat-induced lung injury during long-term follow-up. Korean J Intern Med. 2009. 24:95–100.
15. Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008. 46:515–518.
16. Kim YT, Jou SS, Lee HS, Gil HW, Yang JO, Lee EY, Hong SY. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci. 2009. 24:636–640.
17. Hong SY, Yang JO, Lee EY, Lee ZW. Effects of N-acetyl-L-cysteine and glutathione on antioxidant status of human serum and 3T3 fibroblasts. J Korean Med Sci. 2003. 18:649–654.
18. Collen D, Lijnen HR. Thrombolytic agents. Thromb Haemost. 2005. 93:627–630.
19. Murray V, Norrving B, Sandercock PA, Terént A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010. 267:191–208.
20. Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis- current understanding from an epidemiological point of view. Br J Haematol. 2010. 149:824–833.
21. Kahn SR, Shrier I, Kearon C. Physical activity in patients with deep venous thrombosis: a systematic review. Thromb Res. 2008. 122:763–773.
22. Ortel TL. Acquired thrombotic risk factors in the critical care setting. Crit Care Med. 2010. 38:2 Suppl. S43–S50.
23. Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY. Clinical outcome of hemoperfusion in poisoned patients. Blood Purif. 2010. 30:84–88.
24. Gil HW, Seok SJ, Jeong DS, Yang JO, Lee EY, Hong SY. Plasma level of malondialdehyde in the cases of acute paraquat intoxication. Clin Toxicol (Phila). 2010. 48:149–152.
25. Kim JH, Gil HW, Yang JO, Lee EY, Hong SY. Effect of glutathione administration on serum levels of reactive oxygen metabolites in patients with paraquat intoxication: a pilot study. Korean J Intern Med. 2010. 25:282–287.
26. Thachil J, Fitzmaurice DA, Toh CH. Appropriate use of D-dimer in hospital patients. Am J Med. 2010. 123:17–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download