Abstract
Parasitemia characteristics of Plasmodium vivax malaria in temperate regions may differ from those in tropical zones. However, most parasitological and clinical features of P. vivax malaria have been investigated in the latter. In this study, we investigated 383 malaria patients to clarify the parasitemia characteristics of a P. vivax strain in the Republic of Korea (ROK). The mean parasitemia (8,396/µL) was less than half of tropical P. vivax malaria, and multiple invasions of erythrocytes were not rare (53.5% of the patients, 2.4% of the total investigated RBCs), but less than the observations in tropical zones. The intervals between the first symptom onset and diagnosis were significantly longer in gametocyte (+) patients than in gametocyte (-) patients. Only half of the total patients had both genders of gametocytes (191 of 353), and the male gametocyte density (169/µL) was lower than that of P. vivax strains of a previous study. Multiple invasions of erythrocytes and gametocytemia were coincident factors of the degree of anemia in P. vivax malaria. The present findings demonstrate the P. vivax strain in ROK reveals relatively low parasitemia and low male to female gametocyte ratio. The low ratio may be related with low transmission efficacy.
Malaria is the most prevalent parasitic disease in the world. Each year 300 to 500 million cases occur worldwide, and approximately one to three million people die of malaria (1, 2). Among the four species of human Plasmodium, P. vivax, which causes malaria in temperate zones and also in large areas of tropical regions, is the second most common species. P. vivax malaria was endemic until the early 1970s in the Korean Peninsula (3-5), and re-emerged in the Republic of Korea (ROK) in 1993 (6, 7). Based on epidemiological data, re-emergence of P. vivax malaria in ROK was ascribed to infected mosquitoes originating from North Korea. Since the re-emergence, nearly a million cases of P. vivax malaria have occurred in ROK and North Korea (6, 8-10), and the malaria situation of ROK and North Korea provides a favorable environment to elucidate the parasitemia characteristics of P. vivax malaria. Many parasitemia and clinical features of P. vivax malaria have been investigated so far, but most of the data have been acquired from tropical regions. Transmission and symptom development of P. vivax malaria in temperate zones are largely influenced by seasonal change, whereas such seasonal interruption is less obvious in tropical zones. Therefore, parasitemia characteristics of P. vivax malaria in temperate zones may be quite different from those in tropical zones. However, data from temperate regions have been scarce except those acquired from malaria therapy by artificially induced infection for neurosyphilis patients (11-13). In that therapy, the number of inoculums was higher than in natural infections, which might have resulted in clinical and parasitemia differences from natural infections.
The concentrated occurrence of P. vivax malaria near the Demilitarized Zone between ROK and North Korea provides a good opportunity to better understand the parasitemia characteristics of P. vivax malaria in a temperate zone. Parasitemia characteristics including gametocytemia feature is the important factor related to clinical manifestation and transmission efficacy of Plasmodium. In this study, we investigated the Giemsa-stained peripheral blood smears and clinical laboratory data of P. vivax malaria patients in ROK to clarify the parasitemia characteristics of the P. vivax strain in ROK.
Subjects enrolled in this study were P. vivax malaria patients who were diagnosed during 2002-2006 at six private hospitals located in the northwestern malaria-prevalent areas in ROK. All of the 383 patients were civilians or veterans. The diagnoses were made by microscopic examination of Giemsa-stained peripheral blood smears. Multiple invasion and parasitemia were calculated by microscopic examination of 1,000 thin film fields at 1,000 × magnification. Patients' epidemiological and demographic data were obtained from clinical records. Parasitemia characteristics were elucidated by examination of the patients' peripheral blood smears prepared when the disease was diagnosed. Hematological and serological data at the time of diagnosis were also obtained from clinical records. No patient was undergoing chemoprophylaxis when they visited the hospitals. Any additional examination or blood collection was not performed for the sake of this study and all protocols were approved by the institutional review board of each hospital.
Statistical analyses were performed using the chi-square test without any assumption about the distribution of data using SPSS (Statistical Products and Service Solutions, Chicago, IL, USA). Continuous variables were compared by the Student's t test and Mann-Whitney U tests. Statistical significance was defined as a P value < 0.05.
Of the 383 P. vivax malaria patients enrolled in this study, 283 (74%) were male and the remaining 100 were female (Fig. 1A). Median age of the patients was 39 yr (range, 14-89). More than half of the patients were in their 20 sec (104/383) and 40 sec (102/383) representing 27.2% and 26.6% of the study population, respectively. The next populated age group was in their 30 sec (19.8%, 76/383). Most patients (96%) in this study presented at the hospitals between May and October (Fig. 1B). The mean parasitemia was 8,396/µL with the minimum and the maximum of 12/µL and 140,994/µL, respectively (Table 1). Approximately half of the patients (50.9%) showed parasitemia between 1,000-10,000/µL, with the remainder showing either more than 10,000/µL or less than 1,000/µL.
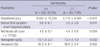
Gametocytes were found in 92.2% of the patients (Table 2). Parasitemias were significantly higher in gametocyte (+) patients than in gametocyte (-) patients. Intervals between the onset of the first symptom and diagnosis were also significantly longer in gametocyte (+) patients (P = 0.001). The anemia markers, red blood cell (RBC) counts, hemoglobin, and hematocrit levels, were significantly higher in gametocyte (-) patients.
Multiple invasions, which refer to infection of one RBC by multiple ring forms, occurred in 53.5% of the patients (Table 3). The intervals between the onset of the first symptom and diagnosis were significantly longer in multiple invasion (+) patients. The anemia markers were significantly higher in multiple invasion (-) patients. Among 205 patients with multiply infected RBCs, 151 patients showed RBCs with only two ring forms, and the maximum number of parasites per RBC was six in a patient. The mean proportion of RBCs infected by multiple ring forms among the total RBCs investigated in this study was 2.4% (with a minimum of 0.19% and a maximum of 11.2%; data not shown), which was less than the value ever observed in tropical zones (14).
Among 353 gametocyte (+) patients, female gametocytes were found in 350 patients and male gametocytes in 194 patients (Table 4). Both female and male gametocytes were found in 191 patients (Group A), whereas only female gametocytes were found in 159 patients (Group B) and only male gametocytes were found in three patients (Group C). The parasitemia was higher in Group A. The parasitemias of Groups A, B and C were 13,541, 4,699, and 2,533/µL, respectively. The gametocytemia was also higher in Group A regardless of the gender of gametocytes. The mean female gametocytemias of Group A and B were 2,063/µL and 595/µL, respectively, and the mean male gametocytemias of Group A and C were 169/µL and 26/µL, respectively. Intervals between the onset of the first symptom and diagnosis of malaria in Group A, of which the parasitemia and the gametocytemia were higher than the other group, were longer than those in Group B and C. The intervals were 9.2, 8.1, and 5.7 days in Groups A, B and C, respectively. During 2002 through 2006 when the patients were enrolled in this study, annual differences of gametocytemia were not significant (data not shown).
Among the Plasmodium that can cause human malaria, P. falciparum has been studied most intensively while P. vivax has received relatively little attention. Moreover, the majority of studies on P. vivax were performed with tropical strains. For investigation of the parasitemia characteristics of P. vivax malaria in temperate regions, the Korean Peninsula is unique in that P. vivax malaria can be studied without confounding interactions with P. falciparum due to the complete absence of P. falciparum in this area. In ROK, P. vivax malaria occurred on a large scale for the past 17 yr (6, 8-10), and chemoprophylaxis with chloroquine and primaquine has been performed in the military since 1997 (15).
The number of cases observed in the 6 hospitals from 2002 to 2006 (383 cases) accounts for 5.4% of the total cases reported in ROK during the same period (7,119 cases). Most of the patients enrolled in this study were civilians without prior exposure to the chemoprophylaxis, providing a good opportunity to investigate the natural parasitemia characteristics without any significant intervention. Interestingly, no child younger than 14 yr was enrolled during this study even though the 6 hospitals providing data were open to all ages. This made our study population distinct from tropical areas where P. vivax malaria was particularly prevalent in children and infants. The main reasons for paucity of children patients in Korea may be unique population structure of Korean rural areas with very low percentages of younger people and low exposure chance to infected mosquitoes in them. Because children are far more populated in urban areas than in rural areas and they travel much less frequently to rural areas than adults do, they have less chance to be exposed to mosquitoes than adults.
The mean parasitemia in this study was 8,396/µL, which is 6.5 times higher than a previous study also performed in ROK (16). In that study, most of the enrolled patients were veterans who had received chemoprophylaxis before being discharged from the military, which might have resulted in suppression of parasitemia. The mean parasitemia of this study, however, was less than a half of P. vivax malaria in tropical zones (17, 18).
Gametocytes were found in most of the patients enrolled in this study. The intervals between the first symptom onset and diagnosis were significantly longer in gametocyte (+) patients than in gametocyte (-) patients, which means that early case detection and treatment are important to block transmission. Parasitemias were significantly higher in gametocyte (+) patients than in gametocyte (-) patients, presumably due to the longer intervals. This result was similar to that of a previous study (13). The significantly lower levels of anemia indices in gametocyte (+) patients might be ascribed to the higher parasitemias, which means that the existence of gametocytes could be a coincident factor of the degree of anemia in P. vivax malaria regardless of their causal relationship. Data in Table 4 show that male gametocytes might appear in the hosts' blood earlier than female gametocytes, which was in agreement with a previous study (12).
Male gametocyte densities have been believed to be critical in the infectivity of P. vivax (19). It might be ascribed to minor population of male gametocytes among total gametocytes. Under this situation, it is more plausible that male gametocyte densities play a key role to determine fertilization efficacy in Anopheles mosquitoes. In this study, the proportion of male gametocyte (+) patients among the enrolled patients and the ratio of male gametocytes to female gametocytes of group A were much lower than other P. vivax strains (12). In Group A, female gametocytes were 12 times more than male gametocytes. This ratio was 3-6 times higher than that of previous studies performed on P. vivax or P. falciparum (12, 20). Under the sex ratio shown in this study, it is difficult that male gametocytes can make enough flagellated gametes to fertilize female gametes to form zygotes. These findings may be one of plausible explanation for low transmission efficacy of the P. vivax strain in ROK. Since the mid-2000s, epidemiological characteristics of P. vivax malaria have been changed in ROK which raises the possibility of change of transmission efficacy (21). It is necessary to monitor gametocytemia annually since gametocytemia is strongly related to transmission efficacy of Plasmodium.
Most of the P. vivax malaria patients in ROK after re-emergence have been primary infection cases without herd immunity against P. vivax, because ROK was malaria-free for more than a decade. The diagnosis of malaria was usually determined at an early stage after illness in ROK since the primary infection cases probably have apparent symptoms and healthcare system has been working well in ROK. Despite the early diagnosis in ROK, gametocytes were found in 92% of the enrolled patients. The situation in North Korea is presumed to be similar to this result, which provides chances of active P. vivax malaria transmission in the Korean Peninsula.
Multiple invasions have been common in P. falciparum malaria, but rarely described in P. vivax malaria (22). In general, P. falciparum malaria shows higher parasitemia than P. vivax malaria, resulting in more mature schizonts, which can subsequently lead to more frequent multiple invasions (23). In this study, however, multiple infected RBCs were observed in as high as 53.5% of the patients although the mean parasitemia was substantially lower than P. falciparum malaria. The ratio of patients with multiple infected RBCs in this study was also much higher than that observed in a tropical strain of P. vivax (24), supporting the previous speculation that multiple invasion in P. vivax malaria might not be directly related to parasitemia but could be a strain-specific characteristic (25). Parasitemias and the proportion of asexual parasites among the total parasites were significantly higher in multiple invasion (+) patients, which was similar to the result of a previous study (23). The parasitemia was significantly higher and the intervals between the first symptom onset and diagnosis were significantly longer in the patients with multiple invasions. The data in Table 3 show that multiple invasion, like gametocytemia, is also a coincident factor of the degree of anemia in P. vivax malaria.
In conclusion, the P. vivax strain in ROK reveals relatively low parasitemia and low male to female gametocyte ratio. The low male to female gametocyte ratio may be related with low transmission efficacy. Early case detection and subsequent treatment are important to block transmission to vector mosquito.
Figures and Tables
Fig. 1
Demographic and epidemiological characteristics of the Plasmodium vivax malaria patients enrolled in this study. Age and gender distribution (A), Monthly and annual numbers (B).

Table 1
Distribution of parasitemia of the Plasmodium vivax malaria patients (N=383) enrolled in this study*

Table 2
Differences in parasitemia and the interval between the first symptom onset and diagnosis according to the occurrence of gametocytes*
ACKNOWLEDGMENTS
The authors thank Ms. Young-A Kim, Ms. Sun-Young Ahn, Ms. Mi-Young Shin and Ms. Ji-Ae Yoo for their technical support.
AUTHOR SUMMARY
Parasitemia Characteristics of Plasmodium vivax Malaria Patients in the Republic of Korea
Ae-Jung Huh, Yee Gyung Kwak, Eu Suk Kim, Kkot Sil Lee, Joon-Sup Yeom, Yong-Kyun Cho, Chang-Seok Kim, and Jae-Won Park
Parasitemia characteristics of Plasmodium vivax malaria in temperate regions may differ from those in tropical zones. However, most parasitological and clinical features of P. vivax malaria have been investigated in the latter. In this study, we investigated 383 malaria patients to clarify the parasitemia characteristics of a P. vivax strain in the Republic of Korea (ROK). The mean parasitemia (8,396/µL) was less than half of tropical P. vivax malaria, and multiple invasions of erythrocytes were not rare (53.5% of the patients, 2.4% of the total investigated RBCs), but less than the observations in tropical zones. The intervals between the first symptom onset and diagnosis were significantly longer in gametocyte (+) patients than in gametocyte (-) patients. Only half of the total patients had both genders of gametocytes (191 out of 353), and the male gametocyte density (169/µL) was lower than the P. vivax strain of a previous study. Multiple invasions of erythrocytes and gametocytemia were coincident factors of the degree of anemia in P. vivax malaria. Results of this study showed the low parasitemia density and considerable gametocytemia of P. vivax malaria prevalent in ROK.
References
1. Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002. 415:680–685.
2. Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007. 77:6 Suppl. 79–87.
3. Hasegawa Y. Malaria in Korea. Chosun Igakkai Zasshi. 1913. 4:53–69.
4. Hankey DD, Jones R Jr, Coatney GR, Alving AS, Coker WG, Garrison PL, Donovan WLWN. Korean vivax malaria. I. Natural history and response to chloroquine. Am J Trop Med Hyg. 1953. 2:958–969.
5. Paik YH, Ree HI, Shim JC. Malaria in Korea. Jpn J Exp Med. 1988. 58:55–66.
6. Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003. 69:159–167.
7. Ree HI. Unstable vivax malaria in Korea. Korean J Parasitol. 2000. 38:119–138.
8. Jun G, Yeom JS, Hong JY, Shin EH, Chang KS, Yu JR, Oh S, Chung H, Park JW. Resurgence of Plasmodium vivax malaria in the Republic of Korea during 2006-2007. Am J Trop Med Hyg. 2009. 81:605–610.
9. Yeom JS, Ryu SH, Oh S, Lee WJ, Kim TS, Kim KH, Kim YA, Ahn SY, Cha JE, Park JW. Status of Plasmodium vivax malaria in the Republic of Korea during 2001-2003. Am J Trop Med Hyg. 2005. 73:604–608.
10. Yeom JS, Kim TS, Oh S, Sim JB, Barn JS, Kim HJ, Kim YA, Ahn SY, Shin MY, Yoo JA, Park JW. Plasmodium vivax malaria in the Republic of Korea during 2004-2005: changing patterns of infection. Am J Trop Med Hyg. 2007. 76:865–868.
11. Eaton P. Susceptibility of red cells to malaria. Am J Trop Med. 1934. 14:431–437.
12. McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. J Parasitol. 2002. 88:521–535.
13. McKenzie FE, Jeffery GM, Collins WE. Gametocytemia and fever in human malaria infections. J Parasitol. 2007. 93:627–633.
14. Simpson JA, Silamut K, Chotivanich K, Pukrittayakamee S, White NJ. Red cell selectivity in malaria: a study of multiple-infected erythrocytes. Trans R Soc Trop Med Hyg. 1999. 93:165–168.
15. Yeom JS, Ryu SH, Oh S, Choi DH, Song KJ, Oh YH, Lee JH, Kim YA, Ahn SY, Yang HY, Cha JE, Park JW. Evaluation of anti-malarial effects of mass chemoprophylaxis in the Republic of Korea Army. J Korean Med Sci. 2005. 20:707–712.
16. Oh MD, Shin H, Shin D, Kim U, Lee S, Kim N, Choi MH, Chai JY, Choe K. Clinical features of vivax malaria. Am J Trop Med Hyg. 2001. 65:143–146.
17. Collins WE, Sullivan JS, Jeffery GM, Williams A, Galland GG, Nace D, Williams T, Barnwell JW. The Chesson strain of Plasmodium vivax in humans and different species of Aotus monkeys. Am J Trop Med Hyg. 2009. 80:152–159.
18. Sinden RE, Gilles HM. Warrell DA, Gilles HM, editors. The malaria parasites. Essential Malariology. 2002. 4th ed. London: Arnold;8–34.
19. Boyd MF, Kitchen SF. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med. 1937. 17:253–262.
20. Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977. 74:1–8.
21. Park JW. Changing transmission pattern of Plasmodium vivax malaria in the Republic of Korea: Relationship with climate change. Environ Health Toxicol. 2011. 26:in press.
22. Hommel M. Warrell DA, Gilles HM, editors. Diagnostic methods in malaria. Essential Malariology. 2002. 4th ed. London: Arnold;35–58.
23. Wang CC. Multiple invasion of erythrocyte by malaria parasites. Trans R Soc Trop Med Hyg. 1970. 64:268–270.
24. Prasad RN, Prasad H, Virk KJ, Sharma VP. Detection of multiple invasion of erythrocytes by Plasmodium vivax. Trop Med Parasitol. 1990. 41:437–438.
25. Hingst HE. Erythrocyte susceptibility to Plasmodium vivax Grassi and Feletti, 1890. Am J Trop Med. 1938. 18:361–372.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download