Abstract
Histologic patterns at tumor margins may be related to prognosis in several malignancies. We investigated tumor aggressiveness with respect to tumor margin histology in patients with papillary thyroid carcinoma (PTC). Five hundred fourteen consecutive patients who underwent surgery for primary PTC between January and July 2009 were assigned to two groups, one with an infiltrative pattern (I-type, n = 347) at tumor margins and one with an expanding pattern (E-type, n = 167). Tumor aggressiveness was assessed by analyzing relationships between these patterns and known prognostic factors. The analysis showed that unfavorable prognostic factors such as tumor multiplicity (P = 0.002), extrathyroidal extension (P < 0.001), lateral neck lymph node metastasis (P < 0.001) and advanced TNM stage (P = 0.001) were significantly more prevalent in patients with I-type PTC than in those with the E-type. Central neck node metastases were more prevalent without statistical significance in the I-type patients (P = 0.376). Tumor margin histology was not related to gender or tumor size. These results suggest that histologic patterns at tumor margins predict aggressiveness in PTC.
Incidence of thyroid cancer is rapidly increasing in Korea (1). Papillary thyroid carcinoma (PTC) is the most common malignancy of the thyroid and most PTCs display relatively low aggressiveness and have a favorable prognosis. Prognostic factors in PTC include age, tumor size, extrathyroidal extension, lymph node metastases, and distant metastases (2-4). Tumor multiplicity and coexisting lymphocytic thyroiditis may also influence the prognosis (5-11), and some PTC variants show characteristic behaviors. Warthin-like variants, for instance, have a favorable prognosis, while tall cell and columnar variants have a less favorable prognosis than typical PTCs (12-15).
Histologic patterns of growth into surrounding stroma characterize the margins of primary tumors as expanding (pushing) or infiltrative (invasive). To our knowledge, however, the prognostic significance of tumor margin histology in patients with PTC has not been determined. For this purpose, we assessed PTC aggressiveness from relationships between growth patterns at tumor margins and factors known to influence the prognosis of PTC patients.
Of the 762 patients who underwent surgery for thyroid cancer at Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine, between January and July 2009, we enrolled 514 (67.5%) for this study. Patients with recurrent or persistent PTC, those who underwent less than total thyroidectomy were excluded. During histological review, patients with specific PTC variants (follicular, diffuse sclerosing, columnar variants, etc.) or encapsulated PTCs were also excluded; therefore only classical papillary carcinoma patients were enrolled in study. While central compartment node dissections were performed prophylactically, lateral neck dissections were performed in patients with clinically proven lateral node metastases. Medical records and pathology reports of patients were retrospectively reviewed.
The histology of tumor margins were reviewed by an experienced endocrine pathologist. Microscopic examinations were performed using formalin-fixed, paraffin embedded tissue sections routinely hematoxylin-eosin stained and assessed at a × 40 and 100 magnification. Tumor margin was defined as the 'transition zone' between the periphery of tumor and normal thyroid stroma. When the features of tumor margin met the following conditions that 1) well-circumscribed and smooth-pushing border around whole tumor, 2) continuous tumor contour with no pointed portion, and 3) tumor invading normal stroma with broad front, they were classified into E-type (expanding type) (Fig. 1). Whereas, the tumor margins that contained the features like 1) uneven and irregular shaped border, 2) presence of spiculated/pointed portion(s) around tumor, and 3) penetration or dissection of normal stroma by tumor cells were classified into I-type (infiltrative type) (Fig. 2). All 514 PTC patients were classified into one of these two groups.
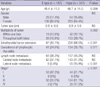
Risk factors, including patient age and gender, size of the primary tumor, tumor multiplicity, extrathyroidal extension, coexistence of lymphocytic thyroiditis, and metastasis to central and/or lateral neck lymph nodes, were compared between patients with E-type margins and those with the I-type. This comparison was also performed for patients with papillary thyroid microcarcinoma (PTMC) (primary tumor size ≤ 1 cm). The distribution of margin types in relation to TNM stage was also evaluated (4).
Mean age of the patients was 47.6 yr (47.6 ± 11.2) and the male to female ratio was 1:5.2 (83:431). There were 167 patients with E-type margins and 347 with I-type margins. All patients underwent total thyroidectomy with central neck node dissection. In addition, 64 patients underwent lateral neck dissection, including 58 patients with unilateral and 6 with bilateral neck dissection. No patients showed distant metastases in preoperative evaluation. The mean age of patients with I-type PTCs was significantly lower than that of patients with E-type tumors (P = 0.009).
Table 1 summarizes the relationships between the types of tumor margin and various clinicopathologic factors. The I-type margin was associated with significantly higher rates of tumor multiplicity (P = 0.002), extrathyroidal extension (P < 0.001), coexistence of lymphocytic thyroiditis (P = 0.011), and metastasis to lateral neck lymph nodes (P < 0.001) than the E-type. The rate of metastasis to central neck lymph nodes was also higher among patients with I-type than with E-type (41.2% vs 37.1%), but the difference was not significant.
Among the 315 patients aged 45 yr or older, we found a significant correlation between advanced stage and I-type margins (P < 0.001) (Table 1). Particularly, all of the 13 patients with T4 stage showed I-type tumor margins. Patients younger than 45 yr (n = 199) were excluded from this analysis because all were in stage I regardless of their T and N status.
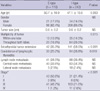
Of our 514 patients, 361 were diagnosed with PTMC. Observing the correlation between clinical or pathologic factors, and margin types of the patients with PTMC, we found significantly higher rates of tumor multiplicity (P = 0.015), extrathyroidal extension (P < 0.001), and coexistence of lymphocytic thyroiditis (P = 0.010) in patients with I-type than with E-type PTMCs (Table 2). The rate of metastasis to central or lateral neck lymph nodes was also higher in patients with I-type than with E-type PTMCs (36.4% vs 34.5%), but the difference was not significant. Among the 230 PTMC patients aged 45 yr or older, we also found a significant correlation between advanced stage and I-type margins (P < 0.001) (Table 2).
Papillary thyroid carcinoma usually has an excellent prognosis, and may be evaluated using various scoring systems. However, 5%-40% of PTC patients have persistent or recurrent disease, with progression and an unfavorable outcome (16, 17). Much effort has therefore been applied to identify and validate new prognostic criteria for PTC.
Since the discovery that infiltrative margins indicate a poor prognosis in colorectal carcinoma, tumor margin histology has been interpreted to indicate tumor aggressiveness or invasiveness. Up to recently, the prognostic significance of this margin type has been approved from several studies in various malignancies (18-22). Including this one, all studies classified the margin types into two categories, (expanding vs infiltrative type) despite of variety of primary tumor; and the criteria for margin classification were also not much different. We thought this categorization was also most suitable and objective in PTC and tried to determine clean and concrete criteria to classify margin types.
The significance of infiltrative margins in PTC was first reported in 1998 (23). That study with 134 patients found a positive association of an infiltrative margin with regional lymph node metastasis of PTC, which suggests that this pattern indicates aggressiveness. We found a strong correlation of the infiltrative margin with unfavorable prognostic factors including tumor multiplicity, extrathyroidal extension and lymph node metastasis. This correlation held also in PTMCs.
There were also some differences between two margin types in patient age and rate of acompanying lymphocytic thyroiditis. We found a higher prevalence of coexistant lymphocytic thyroiditis in patients with I-type tumors than in those with the E-type. In this condition, circulating antibodies to thyroglobulin may prevent tumor spread; however, the prognostic significance of these antibodies, and of lymphocytic thyroiditis per se in PTC requires further study (7-11). And the mean age of patients with I-type tumor margins was lower than in those with the E-type (46.7 vs 49.4 yr). Although prognosis becomes less favorable with age, we did not assign importance to this result because 1) patients with both margin types were more than 45 yr old, and 2) among the patients aged 45 yr or older, significantly more of those with I-type than with E-type tumor margins showed advanced TNM stage (P < 0.001). Hence we concluded that the age difference between these two groups lacked clinical significance.
In this study, we tested only the statistical relationship between tumor margin patterns and prognostic factors previously reported in patients with PTC. To our regret, since these patterns cannot be determined preoperatively, we cannot use them to plan the extent of surgery. However, the strong correlations between tumor margin histology and prognostic factors may inform postoperative treatment. Future studies using larger groups and longer follow-up period are needed to confirm these findings.
Results from this study suggest that relationships between conventional risk factors and histologic patterns at tumor margins may be interpreted to predict tumor aggressiveness in patients with PTC.
Figures and Tables
Fig. 1
Papillary thyroid carcinoma (PTC) with expanding margins (E-type). (A, C, D) PTCs show pushing growth pattern (arrows). No infiltrative portions are identified. (B) Magnification of the squared portion in A (H&E, Magnifications: A and C, × 40; B and D, × 100).

Fig. 2
Papillary thyroid carcinomas (PTC) with infiltrative margins (I-type). (A, B) PTC with extrathyroidal extension (arrowhead). Infiltrative growths into surrounding normal parenchyma are identified (arrows). (C, D) PTC show diffuse, infiltrative margin between tumor and normal parenchyma (arrows). (H&E, Magnifications: A and C, × 40; B and D, × 100).

AUTHOR SUMMARY
Tumor Margin Histology Predicts Tumor Aggressiveness in Papillary Thyroid Carcinoma: A Study of 514 Consecutive Patients
Kuk-Jin Kim, Soon Won Hong, Yong Sang Lee, Bup-Woo Kim, Seung Chul Lee, Hang-Seok Chang, and Cheong Soo Park
Histologic patterns at tumor margins may be related to prognosis in several malignancies. We investigated tumor aggressiveness with respect to tumor margin histology in patients with papillary thyroid carcinoma (PTC). Five hundred fourteen patients who underwent surgery for primary PTC were assigned to two groups, one with an infiltrative pattern (I-type) at tumor margins and one with an expanding pattern (E-type). Tumor aggressiveness was assessed by analyzing relationships between these patterns and known prognostic factors. The analysis showed that unfavorable prognostic factors such as tumor multiplicity, extrathyroidal extension, lateral neck lymph node metastasis and advanced cancer stage were significantly more prevalent in patients with I-type PTC than in those with the E-type. Central neck node metastases were non-significantly more prevalent in the I-type patients. These results suggest that histologic patterns at tumor margins predict aggressiveness in PTC.
References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981. 70:511–518.
3. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990. 71:414–424.
4. Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC cancer staging manual. 2009. 7th ed. New York: Springer-Verlag.
5. Lin YK, Sheng JM, Zhao WH, Wang WB, Yu XF, Teng LS, Ma ZM. Multifocal papillary thyroid carcinoma: clinical analysis of 168 cases. Zhonghua Wai Ke Za Zhi. 2009. 47:450–453.
6. Kim ES, Kim TY, Koh JM, Kim YI, Hong SJ, Kim WB, Shong YK. Completion thyroidectomy in patients with thyroid cancer who initially underwent unilateral operation. Clin Endocrinol (Oxf). 2004. 61:145–148.
7. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, Uchino S, Toda M, Sasaki A, Daa T, Nakayama I. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998. 8:197–202.
8. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999. 84:458–463.
9. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, Hong SJ, Gong G, Shong YK. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009. 71:581–586.
10. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001. 25:632–637.
11. Del Rio P, Cataldo S, Sommaruga L, Concione L, Arcuri MF, Sianesi M. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008. 33:1–5.
12. Johnson TL, Lloyd RV, Thompson NW, Beierwaltes WH, Sisson JC. Prognostic implications of the tall cell variant of papillary thyroid carcinoma. Am J Surg Pathol. 1988. 12:22–27.
13. Moreno Egea A, Rodriguez Gonzalez JM, Sola Perez J, Soria Cogollos T, Parrilla Paricio P. Prognostic value of the tall cell variety of papillary cancer of the thyroid. Eur J Surg Oncol. 1993. 19:517–521.
14. Wenig BM, Thompson LD, Adair CF, Shmookler B, Heffess CS. Thyroid papillary carcinoma of columnar cell type: a clinicopathologic study of 16 cases. Cancer. 1998. 82:740–753.
15. Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: experience at a single institute. Pathology. 2008. 40:617–622.
16. Caron NR, Clark OH. Well differentiated thyroid cancer. Scand J Surg. 2004. 93:261–271.
17. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998. 338:297–306.
18. Jass JR, Love SB, Northover JM. A new prognostic classification of rectal cancer. Lancet. 1987. 1:1303–1306.
19. Rajaganeshan R, Prasad R, Guillou PJ, Chalmers CR, Scott N, Sarkar R, Poston G, Jayne DG. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer. 2007. 96:1112–1117.
20. Zlobec I, Terracciano LM, Lugli A. Local recurrence in mismatch repair-proficient colon cancer predicted by an infiltrative tumor border and lack of CD8+ tumor-infiltrating lymphocytes. Clin Cancer Res. 2008. 14:3792–3797.
21. González-Vela MC, Garijo MF, Fernández FA, Buelta L, Val-Bernal JF. Predictors of axillary lymph node metastases in patients with invasive breast carcinoma by a combination of classical and biological prognostic factors. Pathol Res Pract. 1999. 195:611–618.
22. Barrio AV, Clark BD, Goldberg JI, Hoque LW, Bernik SF, Flynn LW, Susnik B, Giri D, Polo K, Patil S, Van Zee KJ. Clinicopathologic features and long-term outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol. 2007. 14:2961–2970.
23. Mai KT, Perkins DG, Yazdi HM, Commons AS, Thomas J, Meban S. Infiltrating papillary thyroid carcinoma: review of 134 cases of papillary carcinoma. Arch Pathol Lab Med. 1998. 122:166–171.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download