Abstract
To examine the relationship between birth characteristics and childhood cancer mortality, a retrospective cohort study of Korean children was conducted using data collected by the national birth register between 1995 and 2006, which were then individually linked to death data. A cohort of 6,479,406 children was followed from birth until their death or until December 31, 2006. Poisson regression analyses were used to calculate rate ratios of childhood cancer deaths according to birth characteristics. A total of 1,469 cancer deaths were noted and the childhood cancer mortality rate was found to be 3.43 per 100,000 person-years in Korea during the period of 1995-2006. The birth characteristics examined in this study (i.e. , birth weight, gestational age, multiple births, parental ages, and number of siblings) were generally found to be not significantly associated with childhood cancer mortality, and the associations did not vary meaningfully with gender nor with cancer sites. However, among children aged 5-11 yr, higher birth weight was associated with elevated childhood cancer mortality (rate ratio = 1.28, 95% confidence interval 1.04-1.58). Our results offer no overall associations between childhood cancer mortality and birth characteristics, but suggest that the association may be specific to age group.
Cancer is a leading cause of death among children in a majority of nations, with an estimated 161,000 new cancer cases and 87,000 cancer deaths among children aged 14 yr or below reported worldwide in 2007 (1). However, few risk factors have been established for childhood cancer. Only a limited number of environmental factors, such as benzene and ionizing radiation have been strongly associated with the development of childhood leukemia, the most common childhood cancer (2). The fact that childhood cancer is more likely to be triggered by prenatal factors has motivated studies of potential etiological roles of maternal and birth characteristics such as birth weight (3), parental age (4), gestational age (5), multiple births (6), and number of siblings (7). However, several previous investigations into risk factors related to birth characteristics have offered inconsistent results due to the varying ethnicities of study populations, geographical areas involved, study methods, and study periods.
Therefore, our objective was to investigate the association between birth characteristics and both overall and site-specific childhood cancer mortalities by using the national population-based retrospective birth cohort data from 1995 to 2006 in Korea. This study is one of the largest cohort studies exploring relationships between birth characteristics and childhood cancers, enabling the simultaneous examination of a variety of site-specific childhood cancers.
Details of the study design and population have been previously described (8). In brief, data from the national birth and death registration databases of Statistics Korea were used to establish a retrospective cohort of all children born between 1995 and 2006 in Korea. Records from the national birth registration database, based on mandatory parental reporting of birth to Statistics Korea, contain information on each child born (e.g., birth date, gender, area of residence, maternal parity, and personal identification number, along with birth characteristics such as birth weight and gestational age), and on its parents at the time of the birth of the child (e.g., age, marital status, education and occupation).
Of the 6,843,945 total live births between 1995 and 2006, records lacking a complete personal identification number (n = 364,539, 5.3%), an omission primarily due to administrative incompatibilities between the district offices at the place of residence and those at which the birth was registered, were excluded. A total of 6,479,406 records of live births which occurred between 1995 and 2006 in Korea were eventually obtained and linked to the national death registration database for the same period via their unique national personal identification numbers. These individuals were followed from birth until their death or until the end of the follow-up period (December 31, 2006). The individual linkage using personal identification numbers was conducted by Statistics Korea upon our request. For this study we used this merged data minus the personal identification numbers.
The causes of death in the national death register are coded according to the 10th revision of the International Classification of Disease (ICD) (9). We defined cancer deaths as cases marked with ICD codes C00-C97. Cancer deaths were categorized into the following groups: leukemia (C91-C95), central nervous system tumors (C70-C72), adrenal and other endocrine glands tumors (C74-C75), non-Hodgkin's lymphoma (C82-C85), kidney and other urinary organs tumors (C64-C68), liver tumors (C22), soft tissue tumors (C45-C49), eye tumors (C69), bone tumors (C40-C41), and all other cancers.
For birth characteristics, we included gender, birth weight, gestational age, multiple births, parental ages at birth (for both father and mother), and the number of older siblings. The age of the mother at the time of delivery was grouped as ≤ 25 yr, 26-30 yr (reference group), and ≥ 31 yr. Paternal age was grouped as ≤ 30 yr, 31-35 yr (reference group), and ≥ 36 yr. Gestational age was grouped as < 37 weeks, 37-40 weeks (reference group), and ≥ 41 weeks. Birth weight was categorized as ≤ 2,999 g, 3,000-3,499 g (reference group), and ≥ 3,500 g. The number of older siblings was calculated by subtracting one from the maternal parity and grouped as zero (reference group), one, and two or more.
The total number of person-years was calculated by summing the number of days from the date of birth until the date of death or until the completion of the follow-up period (December 31, 2006). Poisson regression analyses were used to estimate rate ratios (RRs) with 95% confidence intervals (95% CIs) of cancer deaths according to birth characteristics. We adjusted for the birth year of the children in all analyses account for any potential cohort effects due to rapid introduction of medical technologies or access to higher-quality medical facilities by birth year. To control for potential confounders, we also included variables for socioeconomic status, such as paternal occupation, maternal education, and location of birth, all of which showed a significant association with childhood cancer death in our previous study (8). In addition, detailed analyses of cancer mortality by gender, age group (< 1, 1-4, 5-11 yr), and types of cancer were conducted separately. We further analyzed cancer mortality after excluding children aged < 1 yr to investigate the consistency of the observed associations. All tests of statistical significance were two-sided, and statistical analyses were performed using Stata 11.0 (StataCorp, College Station, TX, USA).
The cohort contained 42,862,103 person-years of observation, from 1995 through 2006, and a total of 1,469 cancer deaths including 817 (55.6%) boys and 652 (44.4%) girls (Table 1). Leukemia (n = 585, 39.8%) was the leading cause of cancer death, followed by central nervous system tumors (n = 344, 23.4%), adrenal and other endocrine glands tumors (n = 161, 11.0%) and non-Hodgkin lymphoma (n = 62, 4.2%). There were no major gender differences in the distribution of cancer death or in the mortality rate.
Overall, null findings of cancer death in children by birth characteristics were observed compared with their reference groups, except in the cases of girls whose mother aged 25 yr or below (RR = 1.26, 95% CI 1.05-1.53) and boys with a father aged 36 or above (RR = 1.24, 95% CI 1.00-1.53) (Table 2). None of these associations, however, remain significant when the analyses were repeated excluding infants (data not shown). The associations did not meaningfully vary by gender.
Although there were no major differences in the risk estimates by age group, the risk of childhood mortality increased with the highest birth weight (RR = 1.28, 95% CI 1.04-1.58) compared with the reference group among children aged 5-11 yr (Table 3). For children aged < 1 yr, increased risks of cancer death were found for low birth weight (RR = 1.42, 95% CI 1.04-1.93), low gestational age (RR = 2.34, 95% CI 1.46-3.75), and multiple births (RR = 2.16, 95% CI 1.07-4.38). The age-specific results were not meaningfully different between boys and girls (data not shown).
None of the risk estimates between cancer deaths and birth characteristics were significant by cancer site (Table 4). The results were generally consistent across anatomical sites although varying magnitudes of association were observed. When the analyses were repeated excluding infants, the results were essentially similar, with no major gender differences (data not shown). An increased risk for leukemia with low birth weight (RR = 1.43, 95% CI 0.88-2.32) was observed among children aged < 1 yr and with high birth weight among children aged 5-11 yr (RR = 1.46, 95% CI 1.05-2.04) (data not shown).
Making use of nationwide retrospective birth cohort data, we uncovered no overall significant associations between childhood cancer mortality and birth characteristics. However, a small number of birth characteristics were significantly linked with cancer mortality with specific age groups. Among children aged 5-11 yr, high birth weight was associated with an increased risk of cancer death. This is consistent with previous studies that have similarly reported high birth weight to be a risk factor (10). Various mechanisms have been suggested to support the association between high birth weight and cancer risk, such as increased levels of insulin growth factor (11), and the number of bone marrow cells (12). Because birth weight is determined by a variety of factors, including in utero exposures, maternal characteristics, and biological or genetic influences (13), it may be more likely to act as a proxy for multiple factors rather than as an independent cause of childhood mortality.
In contrast to the positive relationship between birth weight and childhood cancer risk among children aged 5-11 yr, a negative relation was observed among the infants in our study. Previous studies have reported that childhood cancer diagnosed in the first year of life has been reported to possess unique characteristics (14) and also showed increased risks of cancer with low birth weight (15). Low birth weight could serve as a surrogate marker of other conditions, such as malnutrition or intrauterine growth-retardation. These may result in severe damage to a fetus which therefore may become more susceptible to other exposures and result in the promotion of cancer deaths, especially in the early phases of life.
In line with previous report (5), we found that children born prior to term are in general not at increased risk for developing childhood cancer. However, in this study children below one year of age showed an increased cancer death risk with shorter gestational age. Preterm birth may be an indicator of certain exposures on the part of the mother, such as tobacco use and/or previous spontaneous abortions, and may also result from in utero exposure to specific diseases and medical procedures (16). Thus, preterm birth may be strongly associated with the subsequent development of cancer death among infants.
Our results indicate that multiple births have no differential effect on the risk of overall cancer death compared with single births, in accord with previous study (17). However, a significantly increased risk associated with multiple births was found in the case of infants. One potential explanation for this finding is the early death of multiple birth neonates, since infants from multiple-fetus pregnancies are generally smaller than those carried alone, and low birth weight in an infant is in turn associated, either directly or indirectly, with childhood mortality. In addition, the in utero environment for multiple gestations may well differ from that of singletons (18).
Although some studies have suggested that the association of birth characteristics with childhood cancer risk differed with the age of children (19), the positive findings observed in this study were mainly limited to infants and may be explained by the high fatality rate in infants. The early onset of childhood cancers is more suggestive of a prenatal genetic origin (20). Cancer deaths triggered by congenital disorders are more likely to affect infants than older children. Furthermore, the rate of survival among infants was relatively lower compared with that of children one to 14 yr of age, although overall childhood cancer survival rates have increased (21). The difference in the association between leukemia and birth weight by the age groups studied may be explained by the delayed immune responses to common exposures associated with birth weight (22). Children with low birth weight whose socioeconomic backgrounds are likely to be substandard might have experienced a particular common exposure (e.g., infection) much earlier than those with higher birth weight, who are generally expected to experience the identical exposure at a later point in their development. However, a more direct indicator used in this study of such exposure (e.g., number of siblings) failed to show similar results.
We found that high paternal and low maternal age were statistically significantly associated with total cancer deaths in boys and in girls, respectively. Certain earlier studies discussed an elevated risk of particular childhood cancers associated with an older parental age at the time of delivery (23), whereas others did not offer evidence of any association between central nervous system malignancies and parental age at birth (24). Older fathers may be more likely to have children with inheritable-mutation disorders (25). Children born to very young mothers may also experience hormonal imbalances and/or inadequate nutrition in utero that could account for our positive findings. However, the positive results became non-significant when we repeated our analysis on children of one year or more of age. Therefore, these findings would be more likely drawn from the high fatality rate of infant cancer cases.
The mortality rate of overall childhood cancer we observed was higher than in the United States (2.69 per 100,000 person-years during 1990-2004) (26) and Japan (boys 2.20, girls 1.89 during 2000-2006) (27), similar to the European Union (boys 3.88, girls 3.24 during 2000-2004) (28), and lower than that seen in urban Shanghai (4.00 for boys, 3.95 for girls during 2003-2005) (29). Although the calendar years and age structures differ, the childhood cancer mortality figures in our study may be comparable to those from other countries.
Certain limitations can be found in this study. First, we were unable to examine whether the relationship between cancer mortality and birth characteristics was due to incidence or survival. This would be especially relevant for leukemia, the leading childhood cancer in this study, where advances in medical treatment have proven to be effective in treating this malignancy. An association with cancer death can be explained by a direct effect of birth characteristics or by one or more confounders, such as socioeconomic status, which may influence access to medical treatment as well as birth characteristics. Although we did conduct analyses excluding infants in order to minimize survival effects and also controlled for certain variables of socioeconomic status during the analyses to minimize the effect of variable access to medical treatment, the potential for residual confounding cannot be excluded. Furthermore, we found the similar distribution of childhood cancer incidence with that of cancer death in general (30). Second, birth and death certificates contain limited information on additional potential confounders such as maternal smoking and conception histories that may well contribute to cancer mortality. Third, a relatively minor proportion of the children (5.3%) were excluded from the study sample due to inexact personal identification numbers. If any association of birth characteristics with cancer mortality turned out to be elevated among those with missing personal identification numbers, then excluding these individuals from the analysis would have resulted in an association toward the null.
Despite such limitations, this study is among the largest studies based on a complete nationwide birth cohort with representativeness for childhood cancer mortality and birth characteristics ever undertaken in Korea. Therefore, the possibility of selection bias is unlikely and we were able to examine some less-common childhood cancers which have not been reported on in other childhood cancer studies. All of the information on birth characteristics was collected prior to outcome ascertainment, subsequently reducing concerns of different recall bias among cases and non-cases.
In conclusion, our results from nationwide retrospective birth cohort data do not generally support a significant role for birth characteristics in deaths from childhood cancers. However, the results differ somewhat by age groups.
Figures and Tables
Table 1
Distributions of childhood cancer deaths by cancer site and gender among children born in Korea, 1995-2006
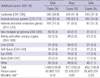
Table 2
Risks of childhood cancer death by gender and birth characteristics among children born in Korea, 1995-2006

AUTHOR SUMMARY
Childhood Cancer Mortality and Birth Characteristics in Korea: A National Population-based Birth Cohort Study
Eun Shil Cha, Kyoung Ae Kong, Eun Kyeong Moon, Young-Ho Khang, and Won Jin Lee
Cancer is a leading cause of death among children, but few risk factors have been established for the childhood cancer. Therefore, to examine the relationship between birth characteristics and childhood cancer mortality, a retrospective cohort study was conducted using data collected by the national birth register between 1995 and 2006 in Korea. Among the cohort of 6,479,406 children, 1,469 cancer deaths were noted (cancer mortality rate, 3.43 per 100,000 person-years). The birth characteristics (i.e. , birth weight, gestational age, multiple births, parental ages, and number of siblings) were generally found to be not significantly associated with childhood cancer mortality. However, among children aged 5-11 yr, higher birth weight was associated with elevated childhood cancer mortality. Our large-scale study suggest the association between childhood cancer and birth characteristics in a specific to age group.
References
1. Global cancer facts and figures 2007. accessed on 1 Sep 2010. American Cancer Society;Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/globalfactsandfigures2007rev2p.pdf.
2. Ross JA, Spector LG. Schottenfeld D, Fraumeni JF, editors. Cancers in children. Cancer epidemiology and prevention. 2006. 3rd ed. New York: Oxford University Press;1251–1268.
3. Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009. 124:2658–2670.
4. Johnson KJ, Carozza SE, Chow EJ, Fox EE, Horel S, McLaughlin CC, Mueller BA, Puumala SE, Reynolds P, Von Behren J, Spector LG. Parental age and risk of childhood cancer: a pooled analysis. Epidemiology. 2009. 20:475–483.
5. Mellemkjaer L, Hasle H, Gridley G, Johansen C, Kjaer SK, Frederiksen K, Olsen JH. Risk of cancer in children with the diagnosis immaturity at birth. Paediatr Perinat Epidemiol. 2006. 20:231–237.
6. Murphy MF, Whiteman D, Hey K, Griffith M, Gill L, Goldacre MJ, Vincent TJ, Bunch K. Childhood cancer incidence in a cohort of twin babies. Br J Cancer. 2001. 84:1460–1462.
7. Altieri A, Castro F, Bermejo JL, Hemminki K. Number of siblings and the risk of lymphoma, leukemia, and myeloma by histopathology. Cancer Epidemiol Biomarkers Prev. 2006. 15:1281–1286.
8. Kong KA, Khang YH, Cha ES, Moon EK, Lee YH, Lee WJ. Childhood cancer mortality and socioeconomic position in South Korea: a national population-based birth cohort study. Cancer Causes Control. 2010. 21:1559–1567.
9. World Health Organization. International statistical classification of diseases and related health problems-tenth revision. 1992. Geneva: World Health Organization.
10. Paltiel O, Harlap S, Deutsch L, Knaanie A, Massalha S, Tiram E, Barchana M, Friedlander Y. Birth weight and other risk factors for acute leukemia in the Jerusalem Perinatal Study cohort. Cancer Epidemiol Biomarkers Prev. 2004. 13:1057–1064.
11. Petridou E, Skalkidou A, Dessypris N, Moustaki M, Mantzoros C, Spanos E, Trichopoulos D. The Childhood Haematologists-Oncologists Group. Endogenous risk factors for childhood leukemia in relation to the IGF system (Greece). Cancer Causes Control. 2000. 11:765–771.
12. Strohsnitter WC, Savarese TM, Low HP, Chelmow DP, Lagiou P, Lambe M, Edmiston K, Liu Q, Baik I, Noller KL, Adami HO, Trichopoulos D, Hsieh CC. Correlation of umbilical cord blood haematopoietic stem and progenitor cell levels with birth weight: implications for a prenatal influence on cancer risk. Br J Cancer. 2008. 98:660–663.
13. Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ. Intrauterine growth and its relationship to size and shape at birth. Pediatr Res. 2002. 52:263–268.
14. Spector LG, Davies SM, Robison LL, Hilden JM, Roesler M, Ross JA. Birth characteristics, maternal reproductive history, and the risk of infant leukemia: a report from the children's oncology group. Cancer Epidemiol Biomarkers Prev. 2007. 16:128–134.
15. Schüz J, Kaletsch U, Kaatsch P, Meinert R, Michaelis J. Risk factors for pediatric tumors of the central nervous system: results from a German population-based case-control study. Med Pediatr Oncol. 2001. 36:274–282.
16. Kim YJ, Lee BE, Park HS, Kang JG, Kim JO, Ha EH. Risk factors for preterm birth in Korea: a multicenter prospective study. Gynecol Obstet Invest. 2005. 60:206–212.
17. Puumala SE, Carozza SE, Chow EJ, Fox EE, Horel S, Johnson KJ, McLaughlin C, Mueller BA, Reynolds P, Von Behren J, Spector LG. Childhood cancer among twins and higher order multiples. Cancer Epidemiol Biomarkers Prev. 2009. 18:162–168.
18. Thomas HV, Murphy MF, Key TJ, Fentiman IS, Allen DS, Kinlen LJ. Pregnancy and menstrual hormone levels in mothers of twins compared to mothers of singletons. Ann Hum Biol. 1998. 25:69–75.
19. Podvin D, Kuehn CM, Mueller BA, Williams M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. 2006. 20:312–322.
20. Spector LG, Hooten AJ, Ross JA. Ontogeny of gene expression: a changing environment for malignancy. Cancer Epidemiol Biomarkers Prev. 2008. 17:1021–1023.
21. Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010. 28:2625–2634.
22. Petridou E, Dalamaga M, Mentis A, Skalkidou A, Moustaki M, Karpathios T, Trichopoulos D. Childhood Haematologists-Oncologists Group. Evidence on the infectious etiology of childhood leukemia: the role of low herd immunity (Greece). Cancer Causes Control. 2001. 12:645–652.
23. Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. Int J Epidemiol. 2006. 35:1495–1503.
24. Mallol-Mesnard N, Menegaux F, Lacour B, Hartmann O, Frappaz D, Doz F, Bertozzi AI, Chastagner P, Hémon D, Clavel J. Birth characteristics and childhood malignant central nervous sytem tumors: the ESCALE study (French Society for Childhood Cancer). Cancer Detect Prev. 2008. 32:79–86.
25. Tarin JJ, Brines J, Cano A. Long-term effects of delayed parenthood. Hum Reprod. 1998. 13:2371–2376.
26. Centers for Disease Control and Prevention (CDC). Trends in childhood cancer mortality--United States, 1990-2004. MMWR Morb Mortal Wkly Rep. 2007. 56:1257–1261.
27. Yang L, Fujimoto J, Qiu D, Sakamoto N. Childhood cancer in Japan: focusing on trend in mortality from 1970 to 2006. Ann Oncol. 2009. 20:166–174.
28. Bosetti C, Bertuccio P, Chatenoud L, Negri E, Levi F, La Vecchia C. Childhood cancer mortality in Europe, 1970-2007. Eur J Cancer. 2010. 46:384–394.
29. Bao PP, Zheng Y, Gu K, Wang CF, Wu CX, Jin F, Lu W. Trends in childhood cancer incidence and mortality in urban Shanghai, 1973-2005. Pediatr Blood Cancer. 2010. 54:1009–1013.
30. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download