Abstract
This study was conducted to evaluate treatment outcome, mortality, and predictors of both in patients with multidrug-resistant tuberculosis (MDR-TB) at 3 TB referral hospitals in the public sector of Korea. We included MDR-TB patients treated at 3 TB referral hospitals in 2004 and reviewed retrospectively their medical records and mortality data. Of 202 MDR-TB patients, 75 (37.1%) had treatment success and 127 (62.9%) poor outcomes. Default rate was high (37.1%, 75/202), comprising 59.1% of poor outcomes. Male sex (adjusted odds ratio [aOR], 2.91; 95% confidence interval [CI], 1.13-7.49), positive smear at treatment initiation (aOR, 5.50; 95% CI, 1.22-24.90), and extensively drug-resistant TB (aOR, 10.72; 95% CI, 1.23-93.64) were independent predictors of poor outcome. The all-cause mortality rate was 31.2% (63/202) during the 3-4 yr after treatment initiation. In conclusion, the treatment outcomes of patients with MDR-TB at the 3 TB hospitals are poor, which may reflect the current status of MDR-TB in the public sector of Korea. A more comprehensive program against MDR-TB needs to be integrated into the National Tuberculosis Program of Korea.
Multidrug-resistant tuberculosis (MDR-TB) is a major challenge for TB control worldwide. Treatment of MDR-TB is difficult because MDR-TB entails higher cost, longer treatment period, and more adverse events than drug-sensitive TB (1, 2). Therefore, a comprehensive control program is essential for MDR-TB. The World Health Organization (WHO) has recommended that the surveillance and management of MDR-TB should be included in the National Tuberculosis Program (NTP) (3).
Korea has a complex TB control system, and the private sector has played a greater role than the public sector in the treatment of TB. In 2008, the notification rate of TB was 77.8% in the private sector and 22.2% in the public sector (4). Since the NTP of Korea has focused on new patients, few data have been reported on the nationwide status of MDR-TB.
In Korea, most MDR-TB patients are treated in 1) the 3 TB referral hospitals in the public sector, 2) the 76 university or tertiary-care hospitals in the private sector, or 3) the 9 Korean National Tuberculosis Association (KNTA) chest clinics, which are considered to be mixed sector (5, 6). The 3 TB hospitals have been responsible for the management of MDR-TB in the public sector of Korea.
The aim of this study was to evaluate treatment outcomes, mortality and predictors of both in MDR-TB patients at the 3 TB hospitals in the public sector of Korea.
Patients diagnosed with MDR-TB and treated at 3 TB hospitals (National Mokpo Tuberculosis Hospital, Mokpo, National Masan Tuberculosis Hospital, Masan, and Seobuk Hospital, Seoul, Korea) between January 1 and December 31, 2004 were included. We reviewed their medical records, radiographic findings, and mortality data retrospectively. If patient was treated for sev eral times, we analyzed the outcome of the first treatment. Mortality data were collected from medical records up to December 31, 2008, or by inquiries made to the Korean National Statistical Office up to December 31, 2007.
MDR-TB is defined as TB resistant to at least isoniazid (INH) and rifampicin (RFP). Extensively drug-resistant TB (XDR-TB) is defined as TB resistant to at least INH and RFP plus any fluoroquinolones and at least one of the injectable second-line drugs (amikacin, kanamycin, or capreomycin) (7).
The patients were classified into the following 3 groups according to their TB treatment history: 1) new patients with no history of TB treatment, 2) patients previously treated with first-line drugs only, or 3) patients previously treated with second-line drugs. Treatment history was defined as treatment with anti-TB drugs for ≥ 30 days.
'Used drug' was defined as a drug that was used for > 3 months. 'Drug with unknown-susceptibility' was defined as a drug used for TB treatment but without drug susceptibility test (DST) (capreomycin, clarithromycin, roxithromycin, amoxicillin-clavulinate, linezolid, interferon-gamma, moxifloxacin and gatifloxacin).
Treatment outcomes were classified according to the WHO's recommended 6 criteria (3) plus "short-term treatment completion". The duration of adequate treatment was defined as ≥ 18 months, including ≥ 12 months after culture conversion. The definition of short-term treatment completion was applied to patients who met all these criteria (6): 1) inadequate treatment duration, but more than 6 months; 2) more than 3 consecutive negative cultures before treatment completion; and 3) treatment completion by a doctor based on favorable treatment response. "Treatment success" was defined as cure, treatment completion and short-term treatment completion. "Poor outcome" was defined as treatment failure, death during treatment, default and transfer out. Patients who interrupted their treatment for ≥ 2 consecutive months were defined as defaulters. Chest radiograph at the time of treatment initiation was categorized based on the National Tuberculosis Association classification (8).
The treatment regimen was individualized based on the most recent DST result and the history of drugs taken by each patient. Injectable agents were typically used for 6 to 7 months. The anti-tuberculosis agents were self-administered except injectable agents during hospitalization. The total treatment duration was a minimum of 18 months after culture conversion. Surgical resection was considered for patients with localized cavitary lesions and anticipated adequate postoperative lung function, and for selected patients with bilateral lesions if medical treatment had failed or was expected to fail.
DSTs were performed at each hospital using the absolute concentration method. Pyrazinamide resistance was determined by pyrazinamidase test in all 3 hospitals.
The chi-square test or Fisher's exact test was performed to compare categorical variables, and Student's t-test or analysis of variance was performed to compare continuous variables. To identify the predictors of poor outcome, we compared variables between treatment success and poor outcome by univariate analysis. Binary logistic regression analysis with backward elimination method was performed for variables with P < 0.2 in the univariate analysis, and the Hosmer-Lemeshow test was used for testing the goodness-of-fit of the models. The Kaplan-Meier method was used for survival analysis, the log-rank test was performed to compare the survival rates between the groups, and Cox regression analysis was performed to identify risk factors associated with mortality. All analysis were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL, USA), and the results with P < 0.05 were considered statistically significant.
In total, 202 patients were included in this study, 46 (23%) from the Seobuk Hospital, 39 (19%) from the National Mokpo Tuberculosis Hospital, and 117 (58%) from the National Masan Tuberculosis Hospital. The mean age was 44.8 (median, 43; range, 16-96) yr and 156 (77.2%) were male (Table 1). HIV-ELISA tests were performed on 2 patients and all were sero-negative. Of 202 patients, 79 (39.1%) had at least 1 comorbidity. Diabetes mellitus was the most common (n = 38, 18.8%), followed by chronic liver disease (n = 12, 5.9%), cardiovascular disease (n = 10, 5.0%), alcohol dependence (n = 9, 4.5%), and psychiatric disease (n = 9, 4.5%).
When patients were categorized on the basis of their previous treatment histories: 41 (21.3%) were new patients, 88 (43.6%) had been treated previously with first-line drugs only, and 73 (36.1%) had been treated previously with second-line drugs. Most of patients were transferred from private clinics and general hospitals (37.6%), public health centers (24.8%), tertiary hospitals (8.9%), and the KNTA chest clinics (8.9%).
A mean of 4.7 (range, 2-9) of 10 tested drugs showed resistance. The baseline resistance patterns are shown in Fig. 1. At treatment initiation, 179 (88.6%) patients had smear-positive sputum. Chest radiograph at the time of treatment initiation showed that 70.1% (141/201) of the patients had far advanced disease, 89.1% (179/201) bilateral disease, 65.2% (131/201) a cavity, and 32.8% (66/201) bilateral cavities.
Treatment regimen included a mean of 4.9 drugs (median 5, range 3-7), of which a mean of 3.6 drugs (median 4, range 0-7) were active by DST. A mean of 0.2 (median 0, range 0-3) 'drugs with unknown-susceptibility' were administered to 29 (14.4%) patients. Injectable agents were administered to 140 (69.3%) patients; streptomycin 85 (60.7%), kanamycin 54 (38.6%), and amikacin 1 (0.7%). Surgical resection was performed on 8 (4%) patients. At treatment initiation, 154 (76.2%) patients were hospitalized.
Among 202 patients, 46 (22.8%) were cured, 17 (8.4%) completed treatment, and 12 (5.9%) completed short-term treatment: therefore, a total of 75 (37.1%) were classified into treatment success (Table 2). The treatment success rate was significantly lower in patients treated with second-line drugs (20.5%, 12/73) than in new patients (53.7%, 22/41; P < 0.001) and patients treated with first-line drugs only (43.2%, 38/88; P = 0.003).
Among 202 patients, 127 (62.9%) had poor outcome; 75 (37.1%) defaulted, 3 (1.5%) failed their treatment, 40 (19.8%) were transferred, and 9 (4.5%) died during treatment. Among the 75 patients who defaulted, 46 (61.3%) were culture positive at the time of treatment interruption and treatment was reinitiated in 12 patients during the study period at the 3 hospitals. Among these 12 re-treated patients, 7 (58.3%) defaulted again, 2 (16.7%) were transferred out, and 1 (8.3%) was cured. Among the 40 transferred patients, 30 (75%) had positive culture at the time of transfer. Mortality rate during treatment was higher in patients previously treated with second-line drugs (9.6%, 7/73) than in new patients (0%, 0/41; P = 0.048) and patients treated with first-line drugs only (2.3%, 2/88; P = 0.08).
The results of univariate analysis for poor outcome are shown in Table 3. Male sex (adjusted odds ratio [aOR], 2.91; 95% confidence interval [CI], 1.13-7.49; P = 0.026), positive smear at treatment initiation (aOR, 5.50; 95% CI, 1.22-24.90; P = 0.027), and XDR-TB (aOR, 10.72; 95% CI, 1.23-93.64; P = 0.032) were independent predictors of poor outcome.
Nine patients died during treatment and additional 54 died during the 3-4 yr of follow-up after treatment initiation, thus all-cause mortality rate was 31.2% (63/202) during the study period. The median survival time of patients previously treated with secondline drugs was significantly shorter than that of new patients (P < 0.001) and patients previously treated with first-line drugs (P = 0.003) (Fig. 2). The median survival time of patients with XDR-TB was significantly shorter than that of patients with non-XDR-TB (P = 0.002).
The results of univariate analysis for predictors of all-cause mortality are shown in Table 4. Independent predictors of all-cause mortality were age (aHR, 1.04; 95% CI, 1.01-1.07; P = 0.006), XDR-TB (aHR, 13.42; 95% CI, 2.98-60.53; P = 0.001), history of MDR-TB treatment (aHR, 2.21; 95% CI, 1.03-4.72, P = 0.042), and resistance to prothionamide (aHR, 3.10; 95% CI, 1.05-9.11; P = 0.040).
This study showed poor outcome for patients with MDR-TB at the 3 TB hospitals in Korea: low treatment success rate (37.1%), high default rate (37.1%), and high all-cause mortality rate (31.2%) during the 3-4 yr after treatment initiation. This result reflects the current status of MDR-TB in the public sector of Korea.
In Korea, the prevalence of TB has decreased markedly since the establishment of NTP in 1962 (9). However, the rate of decrease of TB prevalence has slowed, and MDR-TB has emerged as a significant threat to public health in the 2000s (10, 11). The proportion of MDR-TB among new TB cases increased from 1.6% in 1994 to 2.7% in 2004 (12). Furthermore, Korea has become known as a country with high prevalence of XDR-TB (13). Since the NTP of Korea has focused on new cases, there have been limited nationwide data about the incidence and prevalence of MDR-TB and its treatment outcomes.
Treatment success rate of our study is the lowest ever reported among MDR-TB cohorts in Korea. Treatment success rates of MDR-TB in Korea vary depending on the study sites, range from 44.1% to 66.0% (6, 14-17). It is difficult to determine the current status of MDR-TB in Korea from these studies, since most had a small number of subjects from single institute, different study designs, or different definitions of treatment outcomes. Kim et al. (6) reported the treatment outcomes of 1,407 patients with MDR-TB from 2000 to 2002 in Korea and showed slightly better outcomes than those in our study: 45.3% treatment success rate and 32.2% default rate. The study of Kim et al. is significant for the following reasons. First, the study was conducted on a large numbers of subjects from both the public and private sector. Therefore, it might be more closely reflect the real status of MDR-TB in Korea. Second, their results could be comparable with those in other countries, since the authors mostly followed WHO's recommended cohort analysis. The design of our study was almost the same as that of the study of Kim et al. (6), because we wanted to compare the outcomes by using same definitions.
The treatment success rates of other countries have been reported to be about 62% in 2 meta-analyses (18, 19). The majority of countries included in these meta-analyses had well-established TB control programs such as DOTS (directly observed treatment, short-course)-Plus. This success rate could be a feasible target in Korea if we learn from the experiences of other countries.
The poor outcome in our study is probably related to the high default rate. The default rate in our study was 37.1%, which comprised 59.1% of the poor outcomes. Although it is generally thought that the default rate is higher in the private sector (20), the default rate in our study was not very different from that in the private sector. In the 3 TB hospitals, a nurse or case manager calls patients who default, but this approach allows the tracking of patients only to a limited extent. Default is an issue of MDR-TB management worldwide, but the rate is especially high in Korea, ranging from 3.3% to 39.0% (6, 14-17). The average default rates in other countries have been reported to be 12%-13% (18, 19). High default rates in Korea suggest that the NTP is not effective against MDR-TB.
The lower success rate in our study, compared with other studies in Korea, might be related to a referral bias. The study sites were TB referral hospitals where patients with more severe disease, more comorbidities, and lower socioeconomic status were transferred. In our study, 70.1% of patients had far advanced disease, 36.1% had previously failed to second-line agents, and 47.5% had ofloxacin-resistant strains. Moreover, difference in treatment strategies might influence treatment outcomes. Adjunctive surgical resection is known to be an independent predictor of treatment success (6, 17, 21), but surgical treatment was performed in only 4.0% (8/202) of patients in our study. Further multi-center study including both the private and public sector is needed to clarify the exact reasons for poor outcome in this study.
Inadequate treatment of MDR-TB necessarily results in high mortality and the development of XDR-TB (22), as shown in this study. Independent predictors of poor outcome were male sex, positive smear at treatment initiation, and XDR-TB, which have already been shown to be factors associated with worse outcomes in a meta-analysis (19). XDR-TB was an independent predictor of both poor outcome and all-cause mortality as in the study of Kim et al. (6). XDR-TB is thought to developed and spread due to inappropriate treatment with second-line drugs and lack of adequate infection-control programs. Therefore, XDR-TB is the indicator of inadequate TB control program (23). In this study, the proportion of XDR-TB among new cases was high (17.1%), which suggests that the spread of XDR-TB is presently a serious public health problem in Korea.
Considering the current poor outcomes of MDR-TB, the NTP of Korea should be reevaluated. Comprehensive and aggressive treatment strategies improved treatment outcomes of MDR/XDR-TB (24-26). A comprehensive TB control program should include socioeconomic support, an adequate follow-up system, an infection control program, and careful management of comorbidities, as well as proper case management. DOT is believed to prevent the emergence of drug-resistant TB and has been the essential component of NTP worldwide, since its establishment in 2004 by the WHO (27). Orensteun et al. (18) reported a meta-analysis showing that MDR-TB treatments for more than 18 months and DOT through the entire treatment period are independent predictors of treatment success. DOT is presently conducted in 180 countries (28), but it is not conducted in Korea. Furthermore, social economic support and careful management of comorbidities, such as alcoholism, are other strategies to improve the treatment success rate of TB (24, 29). The lack of such comprehensive strategies, including DOT, might be related to the poor outcomes of MDR-TB in Korea. Although the government of Korea has provided partial support for the medical costs of MDR-TB patients since 2007, more comprehensive management strategy should be integrated into the NTP.
This study has several limitations. First, DSTs were performed in each hospital separately and the qualities of DSTs were not strictly controlled despite their own internal and external quality control program. Second, since DST for amikacin, capreomycin, and other fluroquinolones except ofloxacin were not performed, actual number of XDR-TB patients might be underestimated. Third, HIV-ELISA tests were performed on only 2 patients. Since Korea has been a low HIV-burden country with a reported prevalence below 0.1% (30), the results of our study might reflect that of non-HIV-infected patients with MDR-TB. Despite these limitations, our study is the first study which shows the status of MDR-TB in tuberculosis hospitals in the public sector of Korea.
In conclusion, the treatment outcomes of patients with MDR-TB at the 3 TB hospitals are poor, which may reflect the current status of MDR-TB in the public sector of Korea. A more comprehensive program against MDR-TB needs to be integrated into the NTP of Korea.
Figures and Tables
Fig. 1
Baseline drug resistance among 202 patients with multidrug-resistant tuberculosis. INH, isoniazid; RFP, rifampicin; EMB, ethambutol; PZA, pyrazinamide; SM, streptomycin; KM, kanamycin; OFX, ofloxacin; PAS, para-aminosalicylic acid; PTH, prothionamide; CS, cycloserine.
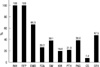
Fig. 2
Kaplan-Meier estimates of survival for 202 patients with multidrug-resistant tuberculosis: (A) all-cause mortality according to previous treatment history (P = 0.032 for a vs b, P = < 0.001 for a vs c, P = 0.003 for b vs c, respectively) and (B) all-cause mortality between XDR-TB and non-XDR-TB (P = 0.002). XDR-TB, extensively drug-resistant tuberculosis.

Table 2
Treatment outcomes of 202 patients with multidrug-resistant tuberculosis according to previous treatment history

AUTHOR SUMMARY
Treatment Outcome and Mortality among Patients with Multidrug-resistant Tuberculosis in Tuberculosis Hospitals of the Public Sector
Doo Soo Jeon, Dong Ok Shin, Seung Kyu Park, Jeong Eun Seo, Hae Sook Seo, Young Soo Cho, Joon Young Lee , Dae Yun Kim, Suck Jun Kong, Yun Seong Kim, and Tae Sun Shim
This study was conducted to evaluate treatment outcome, mortality, and predictors of both in patients with multidrug-resistant tuberculosis (MDR-TB) at 3 TB referral hospitals in the public sector of Korea. We included MDR-TB patients treated at 3 TB referral hospitals in 2004 and reviewed retrospectively their medical records and mortality data. Of 202 MDR-TB patients, 75 (37.1%) had treatment success and 127 (62.9%) poor outcomes. Default rate was high (37.1%, 75/202), comprising 59.1% of poor outcomes. Male sex (adjusted odds ratio [aOR], 2.91; 95% confidence interval [CI], 1.13-7.49), positive smear at treatment initiation (aOR, 5.50; 95% CI, 1.22-24.90), and extensively drug-resistant TB (aOR, 10.72; 95% CI, 1.23-93.64) were independent predictors of poor outcome. The all-cause mortality rate was 31.2% (63/202) during the 3-4 yr after treatment initiation. In conclusion, the treatment outcomes of patients with MDR-TB at the 3 TB hospitals are poor, which may reflect the current status of MDR-TB in the public sector of Korea. A more comprehensive program against MDR-TB needs to be integrated into the National Tuberculosis Program of Korea.
References
1. Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR Jr. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993. 328:527–532.
2. Kang YA, Choi YJ, Cho YJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Cost of treatment for multidrug-resistant tuberculosis in South Korea. Respirology. 2006. 11:793–798.
3. World Health Organization. Publication No. WHO/HTM/TB/2006.361. Guidelines for the programmatic management of drug-resistant tuberculosis. 2006. Geneva, Switzerland: World Health Organization.
4. Korean Centers for Disease Control and Prevention. 2008 Annual Report on the Notified Tuberculosis Patients in Korea. 2009. Seoul: Korean Centers for Disease Control and Prevention.
5. Seung KJ, Bai GH, Kim SJ, Lew WJ, Park SK, Kim JY. The treatment of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2003. 7:912–919.
6. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, Kim EK, Lee KM, Lee SS, Park JS, Koh WJ, Lee CH, Kim JY, Shim TS. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008. 178:1075–1082.
7. Centers for Disease Control and Prevention. Revised definition of extensively drug-resistant tuberculosis. MMWR Morb Mortal Wkly Rep. 2006. 55:1176.
8. National Tuberculosis Association. Diagnostic standards and classification of tuberculosis. 1961. New York: The Association.
9. Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998. 2:27–36.
10. Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Lee NY, Park YK, Bai GH, Koh WJ. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007. 22:677–681.
11. Lee SW, Jeon K, Kim KH, Min KH. Multidrug-resistant pulmonary tuberculosis among young Korean soldiers in a communal setting. J Korean Med Sci. 2009. 24:592–595.
12. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, Lee JK, Kim SJ. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–576.
13. Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006. 55:301–305.
14. Kim HJ, Hong YP, Kim SJ, Lew WJ, Lee EG. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis. 2001. 5:1129–1136.
15. Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004. 8:361–368.
16. Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007. 45:1290–1295.
17. Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, Choi YS, Kim K, Kim J, Shim YM, Koh WJ. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008. 47:496–502.
18. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009. 9:153–161.
19. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009. 4:e6914.
20. Chengsorn N, Bloss E, Anekvorapong R, Anuwatnonthakate A, Wattanaamornkiat W, Komsakorn S, Moolphate S, Limsomboon P, Kaewsaard S, Nateniyom S, Kanphukiew A, Varma JK. Tuberculosis services and treatment outcomes in private and public health care facilities in Thailand, 2004-2006. Int J Tuberc Lung Dis. 2009. 13:888–894.
21. Chan ED, Laurel V, Strand MJ, Chan JF, Huynh ML, Goble M, Iseman MD. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004. 169:1103–1109.
22. Jassal M, Bishai WR. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009. 9:19–30.
23. Raviglione MC, Smith IM. XDR tuberculosis-implications for global public health. N Engl J Med. 2007. 356:656–659.
24. Mitnick CD, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, Fitzmaurice GM, Alcantara Viru FA, Appleton SC, Bayona JN, Bonilla CA, Chalco K, Choi S, Franke MF, Fraser HS, Guerra D, Hurtado RM, Jazayeri D, Joseph K, Llaro K, Mestanza L, Mukherjee JS, Muñoz M, Palacios E, Sanchez E, Sloutsky A, Becerra MC. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008. 359:563–574.
25. Bonilla CA, Crossa A, Jave HO, Mitnick CD, Jamanca RB, Herrera C, Asencios L, Mendoza A, Bayona J, Zignol M, Jaramillo E. Management of extensively drug-resistant tuberculosis in Peru: cure is possible. PLoS ONE. 2008. 3:e2957.
26. Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Atwood S, Mukherjee JS, Rich ML, Furin JJ, Nardell EA, Kim JY, Shin SS. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008. 372:1403–1409.
27. Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC. International standards for tuberculosis care. Lancet Infect Dis. 2006. 6:710–725.
28. World Health Organization. Publication No. WHO/HTM/TB/2009.426. Global tuberculosis control: a short update to the 2009 report. 2009. Geneva, Switzerland: World Health Organization.
29. Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Lomakina OB, Kourbatova EV. Social support and incentives programme for patients with tuberculosis: experience from the Russian Federation. Int J Tuberc Lung Dis. 2007. 11:1210–1215.
30. Choi BY. Perspectives of policies on HIV/AIDS and tuberculosis control in Korea. Korean J Epidemiol. 2006. 28:75–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download