Abstract
Candida haemulonii, one of the non-albicans Candida species, is an emerging yeast pathogen that is known to be resistant to amphotericin B and other antifungal agents such as azoles. These anti-fungal agents have often been associated with clinical treatment failure, so no treatment regimen has been clearly established for invasive C. haemulonii infections. We investigated a catheter-related infection of C. haemulonii candidemia in an adult patient in long-term hospital care. In the early stages, the candidemia remained persistent despite treatment with fluconazole. However, after changing the antifungal agent to caspofungin, the candidemia was resolved. Fluconazole and amphotericin B are not reliable empirical antifungal agents for invasive C. haemulonii infections, as shown in previous case reports. An echinocandin such as caspofungin may be an appropriate empirical choice of antifungal agent for an invasive C. haemulonii infection.
The proportion of candidemia cases caused by non-albicans Candida species has been increasing (1). Some non-albicans Candida species are associated with resistance to the usual antifungal azoles (2). C. haemulonii, a non-albicans Candida species, does not frequently cause human infections (3). Microbiologically, the susceptibility profile of C. haemulonii shows that it is resistant to amphotericin B and other antifungal agents such as azoles (4), which have often been associated with clinical treatment failure (5, 6). No treatment regimen for invasive C. haemulonii infections has been clearly established.
We investigated a catheter-related candidemia infection due to C. haemulonii and report on resolution of the infection using caspofungin, an echinocandin antifungal agent.
On June 25, 2009, a 67-yr-old man with a cerebral infarction was hospitalized in the department of rehabilitation medicine for physical rehabilitation. Prior to admission, he experienced cerebral infarction and was hospitalized for two months, beginning in March, 2009. He had received a tracheostomy at his previous admission.
A physical examination did not reveal any signs of infection, including at the tracheostomy site. The patient received a right subclavian venous catheter for total parenteral nutrition on the second day of hospitalization because of the risk of aspiration when receiving nasogastric tube feedings.
On August 15 (the 50th day of hospitalization), the patient had a temperature of 37.8℃, a white cell count of 10.4 × 109/liter, and a C-reactive protein level of 10.8 mg/liter. However, the blood culture failed to reveal any infectious organism. The mild fever continued, and the level of CRP and white cell count continued to increase.
On August 29 (the 64th day of hospitalization), a blood culture collected 72 hr previously showed yeast growth, and the subclavian venous catheter was removed. Intravenous fluconazole (400 mg once a day) was administered as empirical therapy, pending identification of the yeast. A qualitative culture of the catheter tip also produced a yeast culture.
The yeast from the blood culture and catheter tip, designated as LYS-1, was identified as Candida sp. on the seventh day of fluconazole therapy. The physician continued the fluconazole therapy for the candidemia. The blood cultures performed on the fourth and 14th days of fluconazole therapy were still positive for yeast, and the mild fever persisted.
On September 15 (the 18th day of fluconazole treatment), the infectious disease department was consulted and recommended an echocardiography, a bone scan, an abdominal CT, and Doppler sonography for venous thrombosis. The echocardiograph revealed no signs of infectious endocarditis. The bone scan, abdominal CT, and Doppler sonography revealed no significant findings.
On September 17 (the 84th day of hospitalization), the treatment regimen was changed to caspofungin (50 mg daily after a 70 mg loading dose). The blood culture was sterile on the third and tenth days of caspofungin therapy. On the second day of the caspofungin treatment, the patient became afebrile and showed clinical improvement. In resolution, the patient underwent a 17-day course of caspofungin and remained stable after discontinuation of the antifungal agent.
Conventional automated methods in the clinical microbiology laboratory identified the LSY-1 isolate as Candida sp. Thus, we attempted to identify the culture at the species level using a molecular method. To identify the isolate LSY-1, a portion of the large subunit (LSU) rRNA gene was amplified using the primers LSU-F (5'-GCATATCAATAAGCGGAGGAAAAG-3') and LSU-R (5'-GGTCCGTGTTTCAAGACG-3'). Template DNA and 20 pM of each primer were added to a PCR mixture tube (Accu-Power PCR PreMix; Bioneer, Daejeon, Korea). The reaction mixture was then subjected to 35 cycles of amplification. Each cycle consisted of 30 sec at 95℃, 30 sec at 50℃, and 1 min at 72℃, followed by a final extension at 72℃ for 1 min. The amplified PCR product was purified for sequencing using a PCR purification kit. The purified PCR product was sequenced directly using the same primers as those used in the PCR amplification. The determined sequences (519 bp) were compared with the Gen-Bank public database using the BLASTn program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The LSU rRNA gene sequence of the isolate LSY-1 was a complete match with the corresponding sequence of the reference strain, C. haemulonii strain TJY2d (GenBank accession numbers EU359820). In addition, the isolate in question showed many similarities to the sequences of the C. haemulonii strains and similarities of less than 85% with other Candida species (Fig. 1). Thus, the sample was confidently identified as C. haemulonii.
C. haemulonii was originally described from a sample taken from the gut of a blue-striped grunt (Haemulon scirus) in 1962 (7), and Lavarde et al. (8) reported the first clinical isolation of this fungus from a human in 1984.
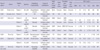
Several meaningful cases of C. haemulonii candidemia have been reported (Table 1). Antifungal resistance is the focus of attention in the management of invasive candidiasis. In previous cases, most C. haemulonii were resistant to both fluconazole and amphotericin B (5, 9). Clinically, when amphotericin B was administered empirically, it failed to eradicate C. haemulonii candidemia (5). The majority of cases in which fluconazole was administered empirically also failed to eradicate candidemia (5, 6). Although fluconazole administration has been shown to resolve candidemia, these cases could be considered transient candidemia combined with the removal of the intravenous catheter. Therefore, the resistance of C. haemulonii poses a therapeutic challenge for the treatment of invasive candidiasis.
Microbiologically, previous reports and our case show that C. haemulonii is susceptible to echinocandins such as caspofungin or micafungin (5, 6, 10), and that it was not resistant to new triazoles such as voriconazole. However, it is not certain that echinocandins or voriconazole are actually efficacious in the treatment of C. haemulonii candidemia. There have been only two cases of clinical success in the eradication of C. haemulonii candidemia via echinocandins administration (5, 6). One case used micafungin, and the other used caspofungin. Of those, in the treatment with caspofungin of a neonate, echinocandin was combined with amphotericin B. Therefore, our case could demonstrate clinical success with caspofungin administration in the eradication of C. haemulonii candidemia in an adult patient.
In our case, the VITEK II (bioMérieux SA) clinical yeast identification system was used for initial identification of C. haemulonii, and sequence analysis of the partial 26s rRNA gene (519 bp) was performed for confirmation. The 519-bp sequences of the isolate matched completely with the corresponding sequences of the reference strain, C. haemulonii strain TJY2d, available in the GenBank database (accession number EU359820). In recent studies, the identification results of the VITEK 2 system have closely corresponded with those of molecular methods for the identification of C. haemulonii, while the VITEK 1 system and the API 32C system usually fail to identify C. haemulonii (5, 10).
Known risk factors for candidemia are use of central-venous catheterization, total parenteral nutrition, previous multiple antibiotics, previous steroid therapy, previous abdominal surgery, and an immunocompromised status (11, 12).
In our case, long-term central-venous catheterization for hyperalimentation was the associated risk factor for candidemia, and no other source of infection suggested the possibility of catheter-related candidemia. In previous case reports, the majority of patients also received an intravenous central line and were in an immunocompromised status.
Considering the recent case series of invasive C. haemulonii infections, C. haemulonii is considered to be an emerging yeast pathogen for which the optimal strategy of patient management has yet to be elucidated.
In conclusion, as found in the previous case reports, fluconazole and amphotericin B are not reliable empirical antifungal agents for the treatment of C. haemulonii candidemia. Echinocandins, such as caspofungin or micafungin, may be an appropriate empirical choice of antifungal agent for invasive C. haemulonii infections. New triazoles require additional clinical testing for treatment of C. haemulonii infection.
Figures and Tables
References
1. Pfaller MA, Diekema DJ. International Fungal Surveillance Participant Group. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin Microbiol Infect. 2004. 10:Suppl 1. 11–23.
2. Vazquez JA, Lundstrom T, Dembry L, Chandrasekar P, Boikov D, Parri MB, Zervos MJ. Invasive Candida guilliermondii infection: in vitro susceptibility studies and molecular analysis. Bone Marrow Transplant. 1995. 16:849–853.
3. Gargeya IB, Pruitt WR, Meyer SA, Ahearn DG. Candida haemulonii from clinical specimens in the USA. J Med Vet Mycol. 1991. 29:335–338.
4. García-Martos P, Domínguez I, Marín P, García-Agudo R, Aoufi S, Mira J. Antifungal susceptibility of emerging yeast pathogens. Enferm Infecc Microbiol Clin. 2001. 19:249–256.
5. Khan ZU, Al-Sweih NA, Ahmad S, Al-Kazemi N, Khan S, Joseph L, Chandy R. Outbreak of fungemia among neonates caused by Candida haemulonii resistant to amphotericin B, itraconazole, and fluconazole. J Clin Microbiol. 2007. 45:2025–2027.
6. Ruan SY, Kuo YW, Huang CT, Hsiue HC, Hsueh PR. Infections due to Candida haemulonii: species identification, antifungal susceptibility and outcomes. Int J Antimicrob Agents. 2010. 35:85–88.
7. Van Uden N, Kolipinski MC. Torulopsis haemulonii nov spec a yeast from the Atlantic Ocean. Antonie Van Leeuwenhoek. 1962. 28:78–80.
8. Lavarde V, Daniel F, Saez H, Arnold M, Faguer B. Peritonite mycosique a Torulopsis haemulonii. Bull Soc Fr Mycol Med. 1984. 13:173–176.
9. Rodero L, Cuenca-Estrella M, Córdoba S, Cahn P, Davel G, Kaufman S, Guelfand L, Rodríguez-Tudela JL. Transient fungemia caused by an amphotericin B-resistant isolate of Candida haemulonii. J Clin Microbiol. 2002. 40:2266–2269.
10. Kim MN, Shin JH, Sung H, Lee K, Kim EC, Ryoo N, Lee JS, Jung SI, Park KH, Kee SJ, Kim SH, Shin MG, Suh SP, Ryang DW. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis. 2009. 48:e57–e61.
11. Hong SK, Nam SH, Kim HC. Fatal peripheral candidal suppurative thrombophlebitis in a postoperative patient. J Korean Med Sci. 2008. 23:1094–1096.
12. Han SS, Yim JJ, Yoo CG, Kim YW, Han SK, Shim YS, Lee SM. Clinical characteristics and risk factors for nosocomial candidemia in medical intensive care units: experience in a single hospital in Korea for 6.6 years. J Korean Med Sci. 2010. 25:671–676.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download