Abstract
Mediastinal lymphadenopathy associated with extrathoracic malignancy or a metastasis of unknown origin (MUO) requires pathological verification. Surgical exploration or endoscopic ultrasound-guided fine needle aspiration is limited to application. We investigated the effectiveness of endobronchial ultrasound-guided transbronchial needle biopsy (EBUS-TBNA) for evaluating mediastinal lymphadenopathy in patients with an extrathoracic malignancy. We retrospectively analyzed data from 59 patients who underwent EBUS-TBNA with a core biopsy because of a suspected mediastinal metastasis between September 2008 and August 2010. All patients had previously been diagnosed with an extrathoracic malignancy (n = 39, 66.1%) or a suspected MUO without a thoracic lesion (n = 20, 33.9%). A total of 88 lymph nodes was analyzed. EBUS-TBNA findings indicated malignancies in 34 patients (57.6%). The EBUS-TBNA sensitivity and specificity for the detection of mediastinal malignancy in patients with a previous extrathoracic malignancy were 96.3% and 100%, respectively. For MUO patients without a thoracic lesion, the sensitivity and specificity were 61.5% and 100%, respectively. The overall sensitivity and specificity were 81.0% and 100%, respectively (P = 0.053). EBUS-TBNA is a safe and effective modality for evaluating mediastinal lymphadenopathy in patients with a previous extrathoracic malignancy or a MUO without a thoracic lesion. The application of this diagnostic tool is likely to have significant clinical implications.
From the construction of a Korea National Cancer Incidence Database for 1999 onward to 2007, the completeness of the Korea Cancer Registry data has improved gradually. This might have contributed in part to the gradual overall increases in cancer incidence, especially among the elderly. The cancer deaths account for about 27% of all deaths in Korea (1, 2).
Patients treated for extrathoracic malignancies can subsequently develop a mediastinal lymphadenopathy. Accurate diagnosis of such mediastinal abnormalities is critical for effective treatment, and a pathological diagnosis should be made if possible (3, 4).
Although mediastinoscopy and open thoracic surgery are standard methods for mediastinal lymph node staging, they are invasive and costly. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is less invasive (5-7) but is limited in terms of access to some nodes and the amount of tissue that can be sampled.
Endobronchial ultrasound-guided needle aspiration (EBUS-TBNA) and biopsy is a minimally invasive procedure that can be used for diagnosing mediastinal lymphadenopathy (8). The procedure shows high sensitivity, specificity, and diagnostic accuracy for evaluating enlarged hilar or mediastinal lymph nodes possibly harboring malignancy (9). EBUS-TBNA has recently been declared a method for lung cancer surgical staging. Few studies have examined the effectiveness of EBUS-TBNA in identifying mediastinal spread in patients with current or previously diagnosed extrathoracic malignancies.
We investigated the effectiveness of EBUS-TBNA for evaluating mediastinal lymphadenopathy in patients diagnosed previously with extrathoracic malignancy or suspected metastatic cancer of unknown primary site (MUO) without a thoracic lesion.
Between September 2008 and August 2010, 777 patients underwent endobronchial ultrasound studies for a variety of clinical indications at the Asan Medical Center. We retrospectively analyzed the data from 59 patients who underwent EBUS-TBNA with core biopsy. The patients underwent EBUS-TBNA because of a suspected mediastinal metastasis according to computed tomography (CT) (short axis > 1 cm) or fluorine 18-labelled deoxyglucose positron emission tomography (F-18 FDG PET). All patients had been diagnosed previously with an extrathoracic malignancy (n = 39, 66.1%) or a suspected MUO without a thoracic lesion (n = 20, 33.9%). There were 40 (67.8%) male patients, and the median age was 62.8 yr (range 20-81 yr).
The EBUS-TBNA procedures were performed by the same interventional pulmonologist. Patients were placed in a conscious sedated state with midazolam. A standard conventional flexible bronchoscopy (model BF-T160 bronchoscope, Olympus, Tokyo, Japan) was first used to examine the tracheobronchial tree. A linear array ultrasonic bronchoscope (XBF-UC 160F; Olympus) with a dedicated 22-gauge needle (NA-202C, Olympus) was subsequently used to perform the ultrasonic examination, transbronchial aspiration and core biopsy. The regional lymph node stations of the mediastinum and hilar regions were imaged systematically and measured (short axis diameter) using the international staging system (Mountain classification). All imaged lymph nodes > 0.5 cm were sampled using real-time ultrasonic needle guidance. Doppler ultrasound was used to identify vessels as necessary. The aspirated material was expelled onto glass slides, smeared, fixed immediately with 95% alcohol, and stained using hematoxylin-eosin (HE) and Papanicolaou stain. Tissue cores (obtained using EBUS-TBNA) were fixed with 10% neutral-buffered formalin and stained using HE. Immunohistochemical staining was also performed when considered necessary. Rapid onsite cytopathological examination was not performed. Patients diagnosed with benign lymphadenopathy by EBUS-TBNA subsequently underwent surgical staging of the mediastinum or clinical and radiological follow-up for at least 6 months.
The primary endpoint was determining the diagnostic yield of EBUS-TBNA. The final diagnoses were based on the pathological findings, clinical symptoms, serological tests, clinical follow-up and, if available, surgical pathology. The sensitivity was compared using the Kendall's tau-b correlation coefficient. A two-tailed P value of less than 0.05 was considered to be significant. Statistical analysis was carried out using the Statistical Package for the Social Sciences (version 14; SPSS Inc., Chicago, IL, USA).
The median mediastinal lymph node size detected with EBUS-TBNA was 15 mm (range 5-50 mm). The median number of needle passages into a node was three (range 1-6). More than half of the EBUS-TBNA samples were from the right lower paratracheal (n = 25, 28.4%) and subcarinal (n = 20, 22.7%) lymph nodes (Table 1). A total of 88 lymph nodes were analyzed using both fine needle aspiration cytology and core biopsy. Three patients had a false negative result in the cytology despite a positive result in the core biopsy. It was possible to determine the origins of metastatic cancers in only seven of 31 patients using the cytology findings. In contrast, the origins could be determined in 30 of 34 patients when using the core biopsy findings. There were no procedure-related complications.
The EBUS-TBNA findings indicated that 34 (57.6%) of the 59 patients were positive for malignancy (Tables 2, 3). Two of these 34 patients were diagnosed with a cancer different from their previous colon cancer: one patient was newly diagnosed with a squamous cell lung cancer, and the other patient was diagnosed with thyroid papillary carcinoma.
The EBUS-TBNA findings indicated that 25 of the 59 patients were negative for malignancy. Four of these 25 patients underwent surgical staging of the mediastinum, which identified mediastinal metastases in one patient, sclerosing mediastinitis in one patient, Castleman's disease in one patient, and a reactive lymph node with negative PET in one patient. Three of the 21 patients underwent excisional biopsy for a neck node. Two of the 21 patients showed progressive mediastinal disease radiologically without tissue confirmation and this was considered tumor positive. For the remaining 19 patients, no mediastinal malignancy was detected during a clinical and radiological median follow-up of 8.1 months (range 2.5-19.8 months).
Ultimately, 40 of the 59 patients were found to have mediastinal metastases using any diagnostic tools. The sensitivity and specificity of EBUS-TBNA for the detection of mediastinal recurrence in patients with a previous extrathoracic malignancy were 96.3% and 100%, respectively. For the detection of mediastinal malignancy in MUO patients, the sensitivity and specificity were 61.5% and 100%, respectively (Table 4). The overall sensitivity and specificity were 81.0%, and 100%, respectively. The sensitivity of EBUS was higher for mediastinal adenopathy with extrathoracic malignant than for MUO (P = 0.053).
This study investigated the effectiveness of EBUS-TBNA in the detection of mediastinum metastases in patients with a suspected mediastinum metastasis who had previously been diagnosed with extrathoracic malignancy or MUO without a thoracic lesion. We found that for the whole study population, EBUS-TBNA had a high diagnostic sensitivity (81%) and specificity (100%). This sensitivity (96.3%) was even higher in the subgroup of patients with a previous extrathoracic malignancy (P = 0.053). There were no complications associated with the use of EBUS-TBNA.
The most important indications for EBUS-TBNA are mediastinal staging for surgical resectability or restaging after chemotherapy or chemoradiation of non-small-cell lung cancer (NSCLC). EBUS-TBNA can also be used to diagnosis a lung mass, mediastinal lymphadenopathy or unknown mediastinopathy, regardless of the presence of an extrathoracic malignancy (8). To date, most studies (10, 11) of EBUS-TBNA have focused on mediastinal staging or restaging, except for the study by Tournoy et al. (12). It is important to diagnose metastases with other causes beside lymphadenopathy such as granulomatous inflammation or infections, and treatable occult malignancies. Chow et al. (13) reported on a case where EBUS-TBNA was used to diagnose an enlarged metastatic mediastinal lymph node caused by an unidentified primary papillary thyroid carcinoma. Hamamoto et al. (14) described a case of epithelioid malignant pleural mesothelioma diagnosed using EBUS-TBNA. After a retrospective chart review, Kennedy et al. (15) reported that 10 of 25 patients with suspected mediastinal recurrences of lymphoma or mediastinal lymphadenopathy of unknown cause were diagnosed with lymphomas using EBUS-TBNA.
EBUS-TBNA is a relatively new modality for sampling mediastinal lymph nodes. Earlier studies of its application in mediastinal staging or restaging of NSCLC showed high diagnostic rates for EBUS-TBNA, with sensitivity and positive predictive values of more than 90% and specificity of 100% (16-18). These values are similar to the present findings. Tournoy et al. (12) reported that sensitivity for mediastinal or hilar metastatic spread of 85%. Kennedy et al. (15) reported that use of EBUS-TBNA to diagnose mediastinal lymphadenopathy in suspected lymphoma cases yielded a high false negative rate (10 of 12 patients). Peric et al. (19) reported that in 75 patients with a previous extrathoracic malignancy and suspected mediastinal metastases, EUS-FNA demonstrated a high sensitivity (86%) (19, 20). Kramer et al. (5) assessed 20 patients with mediastinal lymphadenopathy and previous extrathoracic malignancy, and reported that the sensitivity and specificity of EUS-FNA were 69% and 100%, respectively. In our study, the overall sensitivity and specificity were 81.0%, and 100%, respectively. The sensitivity was higher in non-MUO cases than in MUO cases (96.3% vs 61.5%, P = 0.053).
This is the study to examine the effectiveness of EBUS-TBNA with a focus on mediastinal lymphadenopathy in patients with extrathoracic malignancy. In previous extrathoracic malignancy cased, the sensitivity and specificity of EBUS-TBNA were higher than the figures reported for EUS-FNA (6, 21, 22).
One likely reason for this is that EBUS-TBNA allows access to more lymph nodes compared with EUS-FNA. In addition, EBUS-TBNA samples can undergo histology diagnosis, including immunohistochemistry (23, 24) (Fig. 1). The current findings indicate that EBUS-TBNA was as effective in the present cohort as it is in lung cancer patients.
The present study had several limitations. The study was retrospective in design, involved a relatively small number of patients, and had an unavoidable selection bias.
In conclusion, EBUS-TBNA is found to be a safe, simple and effective procedure. In particular, EBUS-TBNA is effective for assessing mediastinal abnormalities in patients with a history of extrathoracic malignancy. It is concluded that EBUS-TBNA may be a good initial tissue sample test to establish a tissue diagnosis in patients with suspected mediastinal involvement.
Figures and Tables
Fig. 1
Histologic finding on EBUS-TBNA sample using tissue specific immunohistochemical stains. (A) Histologic findings breast cancer (H&E stain, × 100) with (a) positive ER stains (× 200), (B) hepatocelluar carcinoma (H&E stain, ×200) with (b) positive CK stain (×400), (C) malignant melanoma including brownish pigments (H&E stain, × 200) with (c) positive S100 stains (× 400), and (D) renal cell carcinoma cells (H&E stain, × 100) with (d) positive CD10 stains (× 200). EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration.
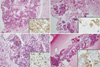
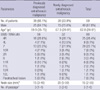
Table 2
Comparison with EBUS-TBNA and final results in patients with a previously diagnosed extrathoracic malignancy

The cancer type of each lymphadenopathy was same between test positive and true positive, except to the case of colon cancer. * were composed of sarcoidosis, thyroid cancer, and no tumor; †were composed of 5 colon cancer and 1 lung cancer. HCC, hepatocelluar carcinoma; RCC, renal cell carcinoma; H&N, head and neck; AGC, advanced gastric cancer; LN, lymph node.
AUTHOR SUMMARY
Endobronchial Ultrasound-guided Transbronchial Needle Biopsy for Diagnosis of Mediastinal Lymphadenopathy in Patients with Extrathoracic Malignancy
Jinkyeong Park, Se Jin Jang, Young Soo Park, Yeon Mok Oh, Tae Sun Shim, Woo Sung Kim, and Chang Min Choi
EBUS-TBNA is a safe and effective modality (81% sensitivity and 100% specificity) for evaluating mediastinal lymphadenopathy in patients with a previous extrathoracic malignancy or a MUO without a thoracic lesion.
References
1. Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, Park EC, Lee JS. Cancer Statistics in Korea: Incidence, Mortality and Survival in 2006-2007. J Korean Med Sci. 2010. 25:1113–1121.
2. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.
3. Mahon TG, Libshitz HI. Mediastinal metastases of infradiaphragmatic malignancies. Eur J Radiol. 1992. 15:130–134.
4. Whooley BP, Urschel JD, Antkowiak JG, Takita H. Primary tumors of the mediastinum. J Surg Oncol. 1999. 70:95–99.
5. Kramer H, Koëter GH, Sleijfer DT, van Putten JW, Groen HJ. Endoscopic ultrasound-guided fine-needle aspiration in patients with mediastinal abnormalities and previous extrathoracic malignancy. Eur J Cancer. 2004. 40:559–562.
6. Fritscher-Ravens A, Sriram PV, Bobrowski C, Pforte A, Topalidis T, Krause C, Jaeckle S, Thonke F, Soehendra N. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000. 95:2278–2284.
7. Kim H, Chung SJ, Kim SG, Kim JS, Jung HC, Song IS. Endoscopic ultrasonography-guided fine needle aspiration for computed tomography-negative and positron emission tomography-positive mediastinal lymph node in a patient with recurrent lung cancer. Gut Liver. 2007. 1:90–92.
8. Cameron SE, Andrade RS, Pambuccian SE. Endobronchial ultrasound guided transbronchial needle aspiration cytology: a state of the art review. Cytopathology. 2010. 21:6–26.
9. Medford AR, Bennett JA, Free CM, Agrawal S. Endobronchial ultrasound guided transbronchial needle aspiration. Postgrad Med J. 2010. 86:106–115.
10. Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008. 26:3346–3350.
11. Lee JE, Kim HY, Lim KY, Lee SH, Lee GK, Lee HS, Hwangbo B. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lung cancer. Lung Cancer. 2010. 70:51–56.
12. Tournoy KG, Govaerts E, Malfait T, Dooms C. Endobronchial ultrasound-guided transbronchial needle biopsy for M1 staging of extrathoracic malignancies. Ann Oncol. 2011. 22:127–131.
13. Chow A, Oki M, Saka H, Moritani S, Usami N. Metastatic mediastinal lymph node from an unidentified primary papillary thyroid carcinoma diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Intern Med. 2009. 48:1293–1296.
14. Hamamoto J, Notsute D, Tokunaga K, Sasaki J, Kojima K, Saeki S, Tanaka R, Tanaka H, Kohrogi H. Diagnostic Usefulness of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in a Case with Malignant Pleural Mesothelioma. Intern Med. 2010. 49:423–426.
15. Kennedy MP, Jimenez CA, Bruzzi JF, Mhatre AD, Lei X, Giles FJ, Fanning T, Morice RC, Eapen GA. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax. 2008. 63:360–365.
16. Nakajima T, Yasufuku K, Iyoda A, Yoshida S, Suzuki M, Sekine Y, Shibuya K, Hiroshima K, Nakatani Y, Fujisawa T. The evaluation of lymph node metastasis by endobronchial ultrasound-guided transbronchial needle aspiration: crucial for selection of surgical candidates with metastatic lung tumors. J Thorac Cardiovasc Surg. 2007. 134:1485–1490.
17. Sun W, Song K, Zervos M, Pass H, Cangiarella J, Bizekis C, Crawford B, Wang BY. The diagnostic value of endobronchial ultrasound-guided needle biopsy in lung cancer and mediastinal adenopathy. Diagn Cytopathol. 2010. 38:337–342.
18. Ernst A, Eberhardt R, Krasnik M, Herth FJ. Efficacy of endobronchial ultrasound-guided transbronchial needle aspiration of hilar lymph nodes for diagnosing and staging cancer. J Thorac Oncol. 2009. 4:947–950.
19. Peric R, Schuurbiers OC, Veseliç M, Rabe KF, van der Heijden HF, Annema JT. Transesophageal endoscopic ultrasound-guided fine-needle aspiration for the mediastinal staging of extrathoracic tumors: a new perspective. Ann Oncol. 2010. 21:1468–1471.
20. Iwashita T, Yasuda I, Doi S, Nakashima M, Tsurumi H, Hirose Y, Takami T, Enya M, Mukai T, Ohnishi T, Iwata K, Tomita E, Moriwaki H. Endoscopic ultrasound-guided fine-needle aspiration in patients with lymphadenopathy suspected of recurrent malignancy after curative treatment. J Gastroenterol. 2009. 44:190–196.
21. Catalano MF, Rosenblatt ML, Chak A, Sivak MV Jr, Scheiman J, Gress F. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am J Gastroenterol. 2002. 97:2559–2565.
22. Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010. 138:790–794.
23. Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, Shibuya K, Iizasa T, Fujisawa T. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005. 50:347–354.
24. Rintoul RC, Skwarski KM, Murchison JT, Wallace WA, Walker WS, Penman ID. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur Respir J. 2005. 25:416–421.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download