Abstract
To confirm the effect of 7-valent pneumococcal conjugate vaccine (PCV7), pneumococcal nasopharyngeal (NP) carriage was compared between vaccinated (3 + 1 doses PCV7) and non-vaccinated children. Vaccinated subjects were recruited from highly vaccinated regions (≥ 60%), Seoul and Incheon whereas control subjects were recruited from Jeju Island where vaccination rates are low (< 15%). NP swabs were obtained from 400 children aged 18-59 months. Serotype and antibiotic susceptibility was analyzed. Pneumococcal carriage rate was 18.0% (36/200) and 31.5% (63/200) for the vaccinated and control group, respectively. Among those vaccinated, 41.7% (15/36) of the serotypes were vaccine-related type (VRT: 6A, 6C, 19A) with the most common serotype 6C. The next common type was non-typable/non-capsule 30.6% (11/36) followed by non-vaccine type 16.7% (6/36) and vaccine type (VT) serotypes were found in only 11.1% (4/36). In contrast, 52.4% (33/63) of the isolates in the control group were VT. Resistance rates for penicillin and erythromycin were lower in the vaccine group (vaccine vs control; penicillin 45.2% vs 71.4%, erythromycin 74.2% vs 90.5%, P < 0.05). Multi-drug resistance was also lower in vaccinated subjects (vaccine vs control; 45.2% vs 69.8%, P < 0.05). PCV7 reduces carriage in VT which leads to replacement of pneumococci by antibiotic susceptible VRT or non-vaccine type strains.
Streptococcus pneumoniae (pneumococcus) is an important cause of meningitis, pneumonia, and bacteremia, especially among young children and older adults (1). Pneumococcal conjugate vaccines were developed to prevent invasive pneumococcal diseases (IPD), and after the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7, Prevenar®, Pfizer Inc, Philadelphia, PA, USA), a substantial decrease in incidence of IPD has been reported in the target population of age < 5 yr (2). PCV7 also showed a herd effect among non-vaccinated populations by reducing nasopharyngeal (NP) colonization and transmission of vaccine-type (VT) pneumococci from vaccinated children (3). Since NP carriage serves as the reservoir of pneumococcal transmission in the community and is the immediate source of IPD in the host (4), many studies have been done about the impact of PCV7 on NP pneumococcal carriage rate (5, 6).
Up to date there are few data on the epidemiology of pneumococcal diseases in Asia and especially about the impact of PCV7 on carriage. In a multicenter, prospective observational study, we studied the effect of PCV7 on pneumococcal NP carriage by comparing the differences in carriage rates, pneumococcal serotypes and antibiotic sensitivity between vaccinated children and non-vaccinated controls.
This study was a multicenter, prospective observational study of the epidemiology of NP carriage. Healthy children aged 18-59 months attending daycare centers for more than 4 hr a day, five days a week in Seoul, Incheon and Jeju Island were recruited. Subjects of the vaccinated group were recruited from Seoul and Incheon and the control group was recruited from Jeju Island. The regional difference in recruitment was due to distinct differences in vaccination rates between the regions. PCV7 is not yet included in the national immunization program and vaccination rates show a difference between regions in Korea; vaccination rates for PCV7 in Seoul and Incheon are more than 60%, whereas the vaccination rate in Jeju Island is lower than 15%. Children fully vaccinated with 3 primary doses and 1 booster dose of PCV7 were enrolled in the vaccinated group and children with no vaccination history of PCV7 were enrolled in the control group from June to December, 2008.
Subjects were excluded if they had 1) febrile illness, 2) present respiratory illness, 3) antibiotics within 7 days before enrollment, 4) immunodeficiency status or 5) nephrotic syndrome.
NP samples were collected from subjects by trained physicians using flexible pediatric calcium alginate swab (Fisher Scientific, Pittsburgh, PA, USA). Swabs were inserted into the posterior nasopharynx, rotated 180 degrees or left in place for > 5 sec, removed and placed in 1.0 mL skim milk or STGG (skim milk, tryptone-glucose-glycerin) transport medium (7). NP swabs were directly transported in ice to the local laboratory, vortexed and frozen at -70℃ until analysis.
After fully thawing the frozen specimen at room temperature, the specimen was mixed thoroughly using a vortex. Each specimen was inoculated onto a 5% defibrinated horse or sheep blood agar plate (including crystal violet and nalidixic acid) and incubated for 24-72 hr at 37℃ in 5% CO2 chamber. Identification of S. pneumoniae was based on the presence of α-hemolysis and inhibition by optochin. For each detected isolate, the serotype and antibiotic susceptibility were determined.
Serotyping was done with the multibead assay method (8) at the University of Alabama at Birmingham, AL, USA. Serotypes were classified as vaccine type (VT; 4, 6B, 9V, 14, 18C, 19F, and 23F), vaccine-related serotype (VRT; 6A, 6C, 6D, and 19A), non-vaccine type (NVT) and non-typable/non-capsulated (NT/NC). PCR was additionally done for isolates classified as 6A to differentiate these isolates from 6C and 6D as previously described (9, 10).
Minimum inhibitory concentration (MIC) was determined by broth microdilution, according to the guidelines of the Clinical and Laboratory Standards Institute (11) at the Catholic University of Korea, Incheon St. Mary's Hospital. In vitro susceptibility was tested for four antimicrobial agents including penicillin (oral penicillin V criteria), amoxicillin, erythromycin and cefaclor.
Differences in carriage rates and serotypes between the vaccinated group and control group were assessed using Fisher's exact test or chi-squared test, as appropriate by SPSS for Windows (version 11.5 software package; SPSS, Chicago, IL, USA). These tests were also used to determine the differences in antibiotic resistance.
The study protocol was approved by the institutional review board at Ewha Womans University Mokdong Hospital (Seoul, Korea), the Catholic University of Korea, Incheon St. Mary's Hospital (Incheon, Korea), and Jeju National University Hospital (Jeju, Korea) (IRB registration number-OCMC08BR013). An informed consent was obtained from parents or guardians of all subjected children before enrollment.
Two-hundred children were enrolled in the vaccinated group and 200 children in the control group. The age (standard deviation) of the subjects in vaccine group and control group was 35.2 (11.6) months and 42.3 (10.5) months, respectively. The male subjects were 47.5% (95/200) in the vaccine group and 53.5% (107/200) in the control group. There was no difference in gender distribution between study group and control group.
Pneumococcal carriage rate was lower in the vaccinated group compared with the control group (18.0% [36/200] vs 31.5% [63/200] [P < 0.05]) (Table 1). There was a distinct difference in proportion of VT and VRT serotypes between the vaccinated and control group (P < 0.05) (Table 1). Among the isolates from vaccinated group, 41.7% (15/36) of the serotypes were VRT (6A, 6C, 19A). The next common serotypes were composed of NT/NC by 30.6% (11/36), 16.7% (6/36) were NVT and only 11.1% (4/36) were VT (6B, 19F, and 23F). Serotype 6C (16.7%, 6/36) was the most common serotype followed by 6A (13.9%, 5/36) in the vaccinated group. Among all vaccinated subjects, the pneumococcal carriage rates for VT and VRT were 2.0% (4/200) and 7.5% (15/200), respectively.
In contrast, a majority of the isolates in the control group were VT by 52.4% (33/63), 14.3% (9/63) of the isolates were VRT and among these serotypes, two of the isolates were determined as 6D. This serotype has recently been discovered from natural pneumococcal isolates in two distant parts of the world, including this study (9, 12). NT/NC isolates were also a large proportion of the control group (22.2%, 14/63). Serotypes 14 (14.3%, 9/63), 6B (12.7%, 8/63), 23F (12.7%, 8/63) and 19F (11.1%, 7/63) were the most common serotypes in the control group. The carriage rates for VT and VRT among all control subjects were 16.5% (33/200) and 4.5% (9/200), respectively.
The antibiotic susceptibility test was done for 31 isolates in the vaccinated group and 63 isolates of the control group. According to the antibiotic susceptibility analysis, regardless of serotype, the resistance rates for penicillin and erythromycin were lower in the vaccine group (vaccine vs control; penicillin 45.2% vs 71.4%, erythromycin 74.2% vs 90.5%, P < 0.05) (Table 2). There was no difference in resistance rate between the vaccine and control group for amoxicillin and cefaclor (vaccine vs control; amoxicillin 22.6% vs 19.0%, cefaclor 96.8% vs 96.8%). Among all pneumococcal isolates, 61.7% (58/94) were found to be multi-drug resistant strains (MDR, resistant to three or more classes of antimicrobial agents) (Table 3). In a comparison between the two study groups, more MDR strains were detected in the control group (vaccine vs control; 45.2% vs 69.8%, P < 0.05).
When the strains were classified according to serotype, all VT strains isolated in vaccinated subjects showed high resistance to penicillin, erythromycin and cefaclor. VT strains isolated in control subjects also showed a penicillin resistance rate of 78.8% (26/33) and erythromycin and cefaclor resistance was noted in almost all VT serotypes. Therefore the proportion of MDR strains among VT isolates were 100% and 78.8% (26/33) in the vaccine and control group, respectively (Table 3).
Regardless of vaccination status, there was an overall high resistant rate for cefaclor for all isolates (66.7%-100%) and for erythromycin (except for NVT strains in vaccinated subjects). However, amoxicillin was sensitive to more than 68.3%-71.0% of the isolates regardless of serotype. When isolates were classified according to serotype group, although more strains were resistant against penicillin for VT serotypes in the vaccine group, the proportion of penicillin resistant strains were lower in the VRT and NVT compared with the control group. The penicillin resistant strains showed a similar proportion in NT/NC strains in both groups.
Up to date, there are few studies about impact of PCV7 to pneumococcal carriage in Asia. This is the first study of pneumococcal carriage among vaccinated children in Korea. We evaluated the impact of PCV7 on NP pneumococcal carriage by comparing differences in carriage rates, pneumococcal serotypes and antibiotic sensitivity between fully vaccinated (3 dose primary series + 1 booster dose) and non-vaccinated control children aged 18-59 months attending daycare centers in Korea. The overall NP pneumococcal carriage rate was higher in the control group compared with the vaccinated group. We also found a significant difference in serotypes according to vaccination status. The carriage rate in the vaccinated population for VT strains was lower compared with control subjects. Also, changes in serotype distribution were seen in comparison with previous studies on pneumococcal carriage in Korea, which were done before the introduction of PCV7 throughout 1998-2001 (13, 14). VT serotypes were approximately 46.6% and 56.9% of the pneumococcal serotypes in those previous studies; these results are similar with the control subjects in this study (52.4%), however a decrease in VT was seen among the vaccinated subjects (11.1%). Changes in serotype distribution such as decrease in VT in vaccinated populations and increase in prevalence of serotypes not covered by the vaccine has been previously reported in other countries also (15-17). These studies reported decrease in VT with an increase in NVT, and stationary prevalence of VRT in vaccinated subjects. The explanation of this finding is based on the hypothesis that PCV7 provides protection against VRT serotypes. However recent reports have shown an increase of VRT including Pn 6C and 19A in NP carriage and IPD isolates (18, 19). Also, it has been stated that PCV7 does not elicit functional antibodies against Pn 19A in infants (20). In this study, we found a higher proportion of VRT isolated in the vaccinated group compared with the control group, with Pn 6C as the most prevalent serotype among the vaccinated subjects. These results are consistent with the recent epidemiologic data questioning the extent of PCV7 cross-protection for particular VRT serotypes such as Pn 6C and 19A.
In this study, Pn 6D was isolated from two subjects in the control group. Following the discovery of serotype 6C, the presence of a new serotype, 6D was proposed but was not found in nature until recently (21). The two 6D isolates described here were found to be 6D based on serologic, genetic, and biochemical characterization (9). Serotype 6D was also recently discovered in Fiji (12). In that study, 86% (12/14) of the subjects from which serotype 6D was isolated, had received at least 1 dose of PCV-7. However, in our study, serotype 6D was detected in children who had no history of vaccination and lived in an area where PCV7 vaccination coverage is low (under 15%). To fully understand the epidemiologic features and characteristics of 6D, further studies will be needed on isolates from sterile sites (IPD), otitis media and NP carriage from different regions.
Another interesting finding was that NT/NC isolates were detected from 30.6% and 22.2% of the vaccinated and control subjects. A previous report of pneumococcal NP carriage before introduction of PCV7 in Korea showed 8% (6/73) of NT isolates (13). Pneumococcal NP carriage serotype acquisition is a complex process with various determinants including age of host, pressure from environment, antibiotics, epidemics, vaccination, capsular switching, competition for predominant serotype or other yet undefined selective forces (22). Up to date there are more than 93 distinct pneumococcal serotypes which are increasing with the development in new methods for detection and serotyping (10, 23, 24). Isolates lacking a capsule rarely cause invasive disease in humans and are attenuated in virulence in animal models of disease (25), however close monitoring on the epidemiologic changes in serotypes of clinical diseases including IPD and otitis media will reveal the significance of this increase in NT/NC isolates.
Antibiotic susceptibility tests showed that penicillin and erythromycin resistance rates were higher in control subjects compared with vaccinated subjects. VT isolates showed a high resistance rate for penicillin, erythromycin and cefaclor in both vaccinated and control subjects. Also, the penicillin resistance rate was higher in control subjects for VRT and NVT strains with a relatively low penicillin resistance rate for NT/NC isolates in both control and vaccinated subjects. Although there are few studies in Korea, we discovered that the total penicillin resistance rate showed a striking increase from 15% in 1999 (26), 27.1% in 2002 (27), 39.7% in 2004 (13) and 71.4% in 2009 (this study) in non-vaccinated subjects compared with a less increase in vaccinated subjects (45.2%, 2009). Therefore with the increase in vaccination of PCV7, decrease in proportion of VT serotypes might lead to a decrease in antibiotic resistance for pneumococcal strains.
According to our findings, resistance rates against cefaclor and macrolide were high regardless of vaccination status or serotype resistance rate. In Korea, erythromycin resistance rates were high (up to 76.3%-88%) among pneumococcal carriage isolates in children 10 yr ago (26, 27).
The overall MDR rate for pneumococcus was 61.7% among all NP carriage isolates regardless of serotype and vaccination status. Although MDR rate was higher in controls compared with vaccinated subjects, increase in vaccination rates of PCV will not be the only solution for this problem. The high antibiotic resistance rate emphasizes the need for policies or guidelines on antibiotic usage to slow done the resistance rate.
There are certain limitations of our study. Samples were collected only a single time from each subject and subjects were recruited throughout summer, autumn and early winter seasons. Pneumococcal carriage rates have been reported to show a significant difference between summer and winter months (28). Considering NP colonization is not a constant process, and pneumococcal isolates and serotypes can differ at different periods of time, a time sequenced sample collection study might give us a better understanding of the local epidemiology. Moreover serotyping was done for a single colony of pneumococcus from each subject. However, pneumococcal serotypes can coexist in the NP of an individual. In studies using methods designed to detect multiple-serotype carriage, the carriage rate for more than one serotype range from 1.3% to 30% (29, 30). Although most studies on the impact of PCV on NP carriage report only the dominant serotype, detection of multiple-strain colonization is also warranted. In the antibiotic sensitivity analysis, isolates were classified by according to serotype such as VT, VRT, NVT or NT/NC. However the antibiotic sensitivity was analyzed for only four VT strains and three NVT strains in the vaccination group. The results of these strains showed a distinct disposition, however it is difficult to make conclusions with this limited data. Also, the subjects of the vaccinated group and control group were recruited from different regions in Korea. The reason for this regional difference in recruitment was due to distinct differences in vaccination rates between the regions. There is also a mild difference in climate between the regions. These factors could influence the pneumococcal carriage rate or serotype distribution. However it is difficult to estimate the impact of these regional differences or differences in background coverage rates on the carriage rate and serotype distribution. Nonetheless, these factors should be considered in interpretation of the data.
Despite these limitations, through a multicenter, prospective observational study, we studied the effect of PCV7 on pneumococcal NP carriage by comparing the differences in carriage rates, pneumococcal serotypes and antibiotic sensitivity between vaccinated children and non-vaccinated controls. This study gives an insight of the impact of PCV7 on the epidemiology of pneumococcal NP carriage in Korean children. With the impressive reduction in carriage of VT serotypes in vaccinated subjects, and considering the high antibiotic resistance rates of these strains, we find that pneumococcal protein conjugate vaccines reduce transmission of these serotypes in the community. Also, the most prevalent serotypes among the vaccinated subjects were VRT and NT/NC. The epidemiologic features regarding the prominent serotype in a community is determined by various environmental factors. PCV7 was introduced in Korea in late 2002 and is still vaccinated on a private sector basis. Continuous monitoring on serotype changes of NP carriage as well as isolates from IPD will be important in making decisions on the national immunization strategy.
Through this study, up to now, we find that PCV7 reduces carriage in VT which leads to replacement of pneumococci by antibiotic susceptible VRT or non-vaccine type strains.
Figures and Tables
Table 1
Serotypes of Streptococcus pneumoniae isolated from vaccinated and non-vaccinated subjects
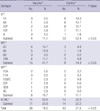
*Total number of subjects, vaccine group (200 subjects), control group (200 subjects); †Carriage rate for serotypes was calculated over the total number of S. pneumoniae strains isolated in the vaccine (n = 36) and control (n = 63) groups. VT, vaccine-type; VRT, vaccine-related type; NVT, non-vaccine type; NT/NC, non-typeable/non-capsule.
Notes
AUTHOR SUMMARY
Nasopharyngeal Pneumococcal Carriage of Children Attending Day Care Centers in Korea: Comparison between Children Immunized with 7-valent Pneumococcal Conjugate Vaccine and Non-immunized
Kyung-Hyo Kim, Jung Yun Hong, Hyunju Lee, Ga Young Kwak, Chan Hee Nam, Soo Young Lee, Eunsang Oh, Jigui Yu, Moon H. Nahm, and Jin Han Kang
To study the impact of the 7-valent pneumococcal conjugate vaccine (PCV7), pneumococcal nasopharyngeal (NP) carriage was evaluated among vaccinated and nonvaccinated children. Among 400 children aged 18-59 months, carriage rate was 18.0% and 31.5% for the vaccinated and control group, respectively. Among those vaccinated, 41.7% of the serotypes were vaccine-related type (VRT: 6A, 6C, 19A) with the most common serotype 6C. The next common type was non-typable/non-capsule 30.6% followed by non-vaccine type 16.7% and vaccine type (VT) serotypes were found in only 11.1%. In contrast, 52.4% of the isolates in the control group were VT. Resistance rates for penicillin and erythromycin were lower in the vaccine group and multi-drug resistance was also lower in vaccinated subjects. We conclude that PCV7 reduces carriage in VT which leads to replacement of pneumococci by antibiotic susceptible VRT or non-vaccine type strains.
References
1. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000. 49:1–35.
2. Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008. 57:144–148.
3. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003. 348:1737–1746.
4. Siber GR, Klugman KP, Makela PH. Pneumococcal Vaccines, The Impact of Conjugate Vaccine. 2008. Washington, DC: AMS Press.
5. O'Brien KL, Millar EV, Zell ER, Bronsdon M, Weatherholtz R, Reid R, Becenti J, Kvamme S, Whitney CG, Santosham M. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007. 196:1211–1220.
6. Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009. 124:e1–e11.
7. O'Brien KL, Nohynek H. World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003. 22:e1–e11.
8. Yu J, Carvalho Mda G, Beall B, Nahm MH. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J Med Microbiol. 2008. 57:171–178.
9. Bratcher PE, Kim KH, Kang JH, Hong JY, Nahm MH. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical, and serological characterization. Microbiology. 2010. 156:555–560.
10. Carvalho Mda G, Pimenta FC, Gertz RE Jr, Joshi HH, Trujillo AA, Keys LE, Findley J, Moura IS, Park IH, Hollingshead SK, Pilishvili T, Whitney CG, Nahm MH, Beall BW. Active Bacterial Core Surveillance Team. PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J Clin Microbiol. 2009. 47:554–559.
11. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. Document M100-S19. 2009. Wayne, PA: Clinical and Laboratory Standards Institute.
12. Jin P, Kong F, Xiao M, Oftadeh S, Zhou F, Liu C, Russell F, Gilbert GL. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J Infect Dis. 2009. 200:1375–1380.
13. Kim SM, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Min AY, Kang JH. Epidemiological study of pneumococcal nasal carriage and serotypes among Korean children. Korean J Pediatr. 2004. 47:611–616.
14. Lee JA, Kim NH, Kim DH, Park KW, Kim YK, Kim KH, Park JY, Choi EH, Lee HJ. Serotypes and penicillin susceptibility of Streptococcus pneumoniae isolated from clinical specimens and healthy carriers of Korean children. J Korean Pediatr Soc. 2003. 46:846–853.
15. Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004. 23:1015–1022.
16. Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996. 348:271–272.
17. Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005. 116:e408–e413.
18. Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009. 199:320–325.
19. Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007. 26:468–472.
20. Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009. 16:376–381.
21. Bratcher PE, Park IH, Hollingshead SK, Nahm MH. Production of a unique pneumococcal capsule serotype belonging to serogroup 6. Microbiology. 2009. 155:576–583.
22. Lipsitch M, Dykes JK, Johnson SE, Ades EW, King J, Briles DE, Carlone GM. Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine. 2000. 18:2895–2901.
23. Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007. 45:1225–1233.
24. Calix JJ, Nahm MH. A new pneumococcal serotype, 11E, has variably inactivated wcjE gene. J Infect Dis. 2010. 202:29–38.
25. Kostyukova NN, Volkova MO, Ivanova VV, Kvetnaya AS. A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol Med Microbiol. 1995. 10:133–137.
26. Park JY, Kim JH. Pharyngeal colonization rate of penicillin-resistant Streptococcus pneumoniae among day-care center children in Seoul, Korea. Korean J Infect Dis. 1999. 31:122–128.
27. Kim KH, Lee JE, Whang IT, Ryu KH, Hong YM, Kim GH, Lee K, Kang ES, Hong KS. Serogroup and antimicrobial resistance of Streptococcus pneumoniae isolated from oropharynx in children attending day care center. J Korean Pediatr Soc. 2002. 45:346–353.
28. Lakshman R, Murdoch C, Race G, Burkinshaw R, Shaw L, Finn A. Pneumococcal nasopharyngeal carriage in children following heptavalent pneumococcal conjugate vaccination in infancy. Arch Dis Child. 2003. 88:211–214.
29. Gratten M, Montgomery J, Gerega G, Gratten H, Siwi H, Poli A, Koki G. Multiple colonization of the upper respiratory tract of Papua New Guinea children with Haemophilus influenzae and Streptococcus pneumoniae. Southeast Asian J Trop Med Public Health. 1989. 20:501–509.
30. Huebner RE, Dagan R, Porath N, Wasas AD, Klugman KP. Lack of utility of serotyping multiple colonies for detection of simultaneous nasopharyngeal carriage of different pneumococcal serotypes. Pediatr Infect Dis J. 2000. 19:1017–1020.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download