Abstract
Since the late 1980s, low dose aspirin has been used to prevent stroke and ischemic heart disease. However, prophylactic effect of antiplatelets against venous thromboembolism (VTE), in patients who undergo hip fracture surgery (HFS) is controversial. Our purpose was to determine the incidence of symptomatic VTE after HFS and to evaluate whether antiplatelets reduce the development of symptomatic VTE following HFS. We retrospectively reviewed 858 HFS in 824 consecutive patients which were performed from May 2003 to April 2010 at an East Asian institute. We compared the incidence of symptomatic VTE in antiplatelet users and non-users using multivariate logistic regression analyses. Overall incidences of symptomatic pulmonary embolism including fatal pulmonary embolism, and symptomatic deep vein thrombosis in this study were 2.4% (21/858), and 3.5% (30/858), respectively. The incidence of symptomatic VTE was 4.8% (12/250) in antiplatelet users and 4.3% (26/608) in non-users (P = 0.718). It is suggested that antiplatelet agents are not effective in prevention of symptomatic VTE after HFS.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major cause of morbidity and mortality after hip fracture surgery (HFS). Without any thromboprophylaxis, the risk of developing DVT following HFS is over 50% and that of developing PE is 7%-11% (1-3). Due to such a high incidence of VTE various thromboprophylactic modalities have been recommended by several guidelines, including the American Academy of Orthopaedic Surgeons (AAOS), the American College of Chest Physicians (ACCP), and National Institute for Health and Clinical Excellence (NICE) (3-5).
In contrast to the large number of studies on developing symptomatic VTE following HFS from Western countries, very few studies have been reported from East Asia.
Daily use of low dose aspirin to prevent thrombosis became popular even in healthy people without any history of cardiovascular disease or cerebrovascular accident since the late 1980s (6, 7). However, thromboprophylactic efficacy of antiplatelets in patients who undergo HFS is controversial (3-5).
The purpose of this study was to determine the incidence of symptomatic VTE after HFS and to evaluate whether antiplatelet users have a low incidence of symptomatic VTE (symptomatic DVT, PE and fatal PE) after HFS than non-users in Korean population.
We retrospectively reviewed the medical records of 851 patients (887 hips), who underwent HFS for femoral neck and intertrochanteric fractures at single institute from May 2003 to April 2010. Twenty-eight patients (29 hips) who were treated with anticoagulant, such as heparin and warfarin; 11 patients (11 hips) due to management for cerebro-vascular disease, 10 patients (11 hips) due to a history of thrombo-embolic event, 6 patients (6 hips) due to a cardiac disease and one patients (one hip) due to femoro-popliteal bypass graft before surgery were excluded, leaving 824 patients for the study.
Of the 824 patients (858 hips), antiplatelet users were defined as those who had taken low-dose aspirin (81-100 mg) or other antiplatelet agents for the prevention of thrombotic cardiovascular or cerebrovascular event before admission for HFS, stopped the antiplatelet medication 5 to 7 days prior to surgery, and resumed medication 1 day to 3 days after the surgery. Two hundred and forty five patients (250 hips) were identified as antiplatelet users. The remaining 579 patients (608 hips) were classified as non-users.
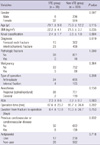
In the antiplatelet users, there were 173 women and 72 men with a mean age of 77.6 yr at the time of operation. Their mean body mass index (BMI) was 22.1 kg/m2. In the non-users, there were 414 women and 165 men with a mean age of 75.2 yr at the time of operation. Their mean BMI was 21.3 kg/m2 (Table 1).
No pharmacological or mechanical prophylaxis for VTE other than antiplatelet was done postoperatively in both groups. However, thigh-length antiembolic stockings were applied and the ankle pump was encouraged in bed during the hospitalization.
One day to three days after the operation, closed suction drain-age was removed and patients were mobilized with wheelchairs. Patients walked with restricted weight-bearing and use of assistive devices (wheelchair, walker, crutches, or cane) 3 to 10 days after the operation. As their walking ability improved, their assistive devices were changed appropriately by physical therapists.
After the operation, we carefully monitored clinical signs of DVT including pain and tenderness in the calf or thigh, swelling or erythema of the operated limb, and a positive Homans' sign. When VTE was suspected, we consulted the patients to cardiovascular physicians. A diagnosis of DVT was made using duplex ultrasonography or lower extremity CT angiography. A PE was confirmed by a ventilation/perfusion scan or pulmonary CT angiography. Patients who were diagnosed as having a DVT or PE were treated with warfarin.
Most deaths due to PE related to the operation reportedly occur within 3 months and any death of unknown cause that occurred within 3 months of operation is considered to be the result of PE (8-10). We confirmed the fatal PE, if present, from the death certificate.
Patients were monitored for 1 week to 3 weeks in the ward. After discharge, patients were routinely followed at 6 weeks, 3 months and 6 months after the operation with a specific interest of VTE. Patients who had not returned on regularly scheduled visits were contacted by telephone.
We compared the incidence of symptomatic VTE (DVT, PE and fatal PE) in both groups. We also assessed gender, age, BMI, ambulatory ability before injury according to Koval's categories (11), type of fracture (femur neck fracture or intertrochanteric fracture), pathologic fracture, malignancy, type of surgery (arthroplasty or internal fixation), type of anesthesia (regional or general), co-morbidity assessed by American Society of Anesthesiologists (ASA) score, operation time, the length of time from fracture to surgery, and previous history of cerebrovascular or cardiovascular disease to determine the relationships between these variables and the development of VTE.
Statistical analyses were planned to determine whether the perioperative use of antiplatelets reduced the incidence of symptomatic VTE. All HFS were divided into 2 groups; the VTE group (symptomatic DVT, PE as well as fatal PE) and non-VTE group. To determine confounding factors, univariate comparisons between the VTE group and the non-VTE group were made based on the demographic data and operative parameters. Statistical significance of the differences between the 2 groups was determined by chi-square test for categorical variables and Student's t-test for continuous variables. For the variables with a P value less than 0.1 in the univariate analyses, multivariate logistic regression analyses using the enter method were performed. In the multivariate logistic regression, the dependent variable was whether the VTE occurred or not postoperatively. From the multivariate regression analyses, it was assessed whether use of antiplatelet statistically reduced occurrence of VTE. Statistical analyses were conducted with the SPSS for Windows statistical package (version 12.0; SPSS, Chicago, IL, USA), and P value less than 0.05 was considered as significant.
Among the 858 procedures, there were 4 deaths of unknown cause within 3 months after the HFS. Symptomatic DVT occurred in 30 patients. Of them, 13 patients suffered symptomatic PE. Four patients suffered symptomatic PE without DVT. Overall incidences of symptomatic PE including fatal PE, and symptomatic DVT in this study were 2.4% (21/858), and 3.5% (30/858), respectively. The incidence of symptomatic VTE was 4.8% (12/250) in antiplatelet users and 4.3% (26/608) in non-users (P = 0.718). In the univariate comparisons, only gender showed P value less than 0.1 (Table 2).
Multivariate logistic regression analyses for the variables with a P value less than 0.1 in the univariate analyses demonstrated that no variable was significantly associated with the VTE. Besides use of antiplatelet, the independent variables tested for the multivariate logistic regression analyses included age, gender, and BMI, as confounding factors (Table 3). The value of R2 coefficient for this multivariate regression model was 0.009, suggesting that this multivariate model would explain the variation of the outcome variable to the extent of 0.9%.
After multivariate logistic regression analyses, use of antiplatelet was not associated with symptomatic VTE in HFS patients (P = 0.841).
In this study, the overall incidence of symptomatic VTE following HFS was 4.4% (38/858), and the perioperative use of antiplatelet did not reduce the incidence of symptomatic VTE after HFS in Korean patients.
Pulmonary Embolism Prevention (PEP) study, which compared the efficacy of low-dose aspirin and placebo in patients undergoing HFS, presented that the aspirin reduced the risk of VTE (12). However, the symptomatic VTE after HFS was not reduced in Korean patients who received antiplatelet agent including aspirin in this study.
On the other hand, the 4.4% overall incidence of VTE in this study is lower than that (46%-60%) after HFS without thromboprophylaxis in Western patients, and comparable with that (1.3%-6%) after HFS in Western patients, who underwent routine thromboprophylaxis (1, 12-16). Although several risk factors for VTE have been presented by previous studies, there was no significant risk factor of VTE in HFS after multivariate analysis in this study.
In terms of HFS, recent Asian studies suggested that the incidence of VTE in Asian patients was similar to that reported from the West (17-21) (Table 4). However, the majority of these studies mainly focused on other procedure such as total knee arthroplasty and total hip arthroplasty, and had too small size of HFS. In addition, most studies used data collected from various areas of Asia and included multiple ethnic groups other than East Asian. In the AIDA study, which evaluated asymptomatic VTE in 837 Asian patients undergoing orthopedic surgery in 19 centers in 7 Asian countries, the rate of asymptomatic DVT in 96 HFS without thromboprophylaxis was 42.0%, which was similar to that from West (Table 4) (20).
Although the incidence (4.4%) of symptomatic VTE in this study was lower than those of the Western studies, our results do not indicate that thromboprophylaxis after HFS is not necessary in Korean patients, because the incidence of symptomatic VTE will always be markedly lower than those of asymptomatic VTE, that are venographically proved. In contrast to elective total hip arthroplasty, the patients with HFS were extremely old and had several co-morbidities such as diabetes and cardiovascular diseases. If these patients suffered symptomatic VTE, their recovery of ambulatory ability prior to injury would be delayed during rehabilitation, and the mortality of the patients with osteoporotic hip fracture would be increased (9, 22).
This study has several limitations. First, our study was not a prospective but a retrospective study. However, a prospective randomized comparative study is barely possible in these senile patients who have a lack of perception on participation. Second, we could not perform VTE studies in asymptomatic patients and did not determine the incidence of asymptomatic VTE. However, VTE studies are associated with procedure-related complications and high medical cost (23, 24), and objectives of prophylaxis in VTE are to prevent fatal PE and to reduce the symptomatic morbidity associated with VTE (25). Therefore, our study on symptomatic VTE in patients with HFS might be justified. Third, subgroup analyses according to antiplatelet agents and their doses were not performed because our patients were treated with various agents and the number of symptomatic patients was small. Fourth, we did not evaluate the patients' compliance in terms of the antiembolic stockings and the ankle pump, which might be a confounding factor for VTE development.
Although antiplatelet therapy has been shown to reduce the risk of arterial occlusive disease (6, 7, 26), it is controversial whether the antiplatelet agents are effective or not in the prevention of VTE (12, 27, 28). It might be argued that the antiplatelet users in our study might have a previous history of major arterial occlusive diseases including cerebrovascular or cardiovascular disease and were more vulnerable to VTE. However, univariate analysis in our study showed that previous arterial disease was not associated with an increased risk of VTE.
The incidence of hip fracture will increase in accordance with prolonged life expectancy in East Asia (29, 30), and VTE after HFS appears as an important medical issue. Our study showed that antiplatelets are not effective in the prevention of symptomatic VTE after HFS, although the incidence of symptomatic VTE after HFS was low. The data of current study could be used for the thromboprophylactic guideline of in Korean patients with HFS.
Figures and Tables
AUTHOR SUMMARY
Little Impact of Antiplatelet Agents on Venous Thromboembolism after Hip Fracture Surgery
Hyung-Min Ji, Young-Kyun Lee, Yong-Chan Ha, Ki-Choul Kim and Kyung-Hoi Koo
We investigated the incidence of symptomatic venous thromboembolism after hip fracture surgery, and evaluated whether antiplatelets reduce the development of symptomatic venous thromboembolism following hip fracture surgery. The incidence of symptomatic VTE was 4.8% (12/250) in antiplatelet users and 4.3% (26/608) in non-users (P = 0.718). It is suggested that antiplatelet agents are not effective in prevention of symptomatic VTE after HFS.
References
1. Todd CJ, Freeman CJ, Camilleri-Ferrante C, Palmer CR, Hyder A, Laxton CE, Parker MJ, Payne BV, Rushton N. Differences in mortality after fracture of hip: the east Anglian audit. BMJ. 1995. 310:904–908.
2. Zahn HR, Skinner JA, Porteous MJ. The preoperative prevalence of deep vein thrombosis in patients with femoral neck fractures and delayed operation. Injury. 1999. 30:605–607.
3. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004. 126:338S–400S.
4. Haas SB, Barrack RL, Westrich G, Lachiewicz PF. Venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2008. 90:2764–2780.
5. Hill J, Treasure T. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients having surgery: summary of NICE guidance. BMJ. 2007. 334:1053–1054.
6. Antiplatelet Trialists' Collaboration. Secondary prevention of vascular disease by prolonged antiplatelet treatment. Br Med J (Clin Res Ed). 1988. 296:320–331.
7. Fuster V, Adams PC, Badimon JJ, Chesebro JH. Platelet-inhibitor drugs' role in coronary artery disease. Prog Cardiovasc Dis. 1987. 29:325–346.
8. Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE, Spera MA, Alvavi A, Terrin ML. The clinical course of pulmonary embolism. N Engl J Med. 1992. 326:1240–1245.
9. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995. 77:6–10.
10. Wroblewski BM, Siney PD, White R. Fatal pulmonary embolism after total hip arthroplasty. Seasonal variation. Clin Orthop Relat Res. 1992. 276:222–224.
11. Koval KJ, Aharonoff GB, Rosenberg AD, Bernstein RL, Zuckerman JD. Functional outcome after hip fracture. Effect of general versus regional anesthesia. Clin Orthop Relat Res. 1998. 37–41.
12. Pulmonary Embolism Prevention (PEP) Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000. 355:1295–1302.
13. Eriksson BI, Lassen MR. PENTasaccharide in HIp-FRActure Surgery Plus Investigators. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003. 163:1337–1342.
14. Rosencher N, Vielpeau C, Emmerich J, Fagnani F, Samama CM. ESCORTE Group. Venous thromboembolism and mortality after hip fracture surgery: the ESCORTE study. J Thromb Haemost. 2005. 3:2006–2014.
15. Westrich GH, Rana AJ, Terry MA, Taveras NA, Kapoor K, Helfet DL. Thromboembolic disease prophylaxis in patients with hip fracture: a multimodal approach. J Orthop Trauma. 2005. 19:234–240.
16. Lieberman DV, Lieberman D. Proximal deep vein thrombosis after hip fracture surgery in elderly patients despite thromboprophylaxis. Am J Phys Med Rehabil. 2002. 81:745–750.
17. Atichartakarn V, Pathepchotiwong K, Keorochana S, Eurvilaichit C. Deep vein thrombosis after hip surgery among Thai. Arch Intern Med. 1988. 148:1349–1353.
18. Mitra AK, Khoo TK, Ngan CC. Deep-vein thrombosis following hip surgery for fracture of the proximal femur. Singapore Med J. 1989. 30:530–534.
19. Dhillon KS, Askander A, Doraismay S. Postoperative deep-vein thrombosis in Asian patients is not a rarity: a prospective study of 88 patients with no prophylaxis. J Bone Joint Surg Br. 1996. 78:427–430.
20. Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, Turpie AG, Gallus AS, Planès A, Passera R, Rouillon A. AIDA Investigators. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005. 3:2664–2670.
21. Bagaria V, Modi N, Panghate A, Vaidya S. Incidence and risk factors for development of venous thromboembolism in Indian patients undergoing major orthopaedic surgery: results of a prospective study. Postgrad Med J. 2006. 82:136–139.
22. Cordell-Smith JA, Williams SC, Harper WM, Gregg PJ. Lower limb arthroplasty complicated by deep venous thrombosis. Prevalence and subjective outcome. J Bone Joint Surg Br. 2004. 86:99–101.
23. Albrechtsson U, Olsson CG. Thrombotic side-effects of lower-limb phlebography. Lancet. 1976. 1:723–724.
24. Bettmann MA, Robbins A, Braun SD, Wetzner S, Dunnick NR, Finkelstein J. Contrast venography of the leg: diagnostic efficacy, tolerance, and complication rates with ionic and nonionic contrast media. Radiology. 1987. 165:113–116.
25. Kakkar VV, Stringer MD. Prophylaxis of venous thromboembolism. World J Surg. 1990. 14:670–678.
26. Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. II: Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994. 308:159–168.
27. Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ. 1994. 309:1213–1215.
28. Sors H, Meyer G. Place of aspirin in prophylaxis of venous thromboembolism. Lancet. 2000. 355:1288–1289.
29. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997. 7:407–413.
30. Lau EM, Lee JK, Suriwongpaisal P, Saw SM, Das De S, Khir A, Sambrook P. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS). Osteoporos Int. 2001. 12:239–243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download