Abstract
We report a case of early non-invasive diagnosis of acute eosinophilic myopericarditis (AEM) by cardiovascular magnetic resonance (CMR) before cardiac biopsy. A 35-yr-old woman presented with a flu-like illness, followed by pleuritic chest pain and shortness of breath. Transthoracic echocardiography revealed mild left ventricular (LV) systolic dysfunction with borderline LV wall thickness and moderate pericardial effusion. The patient had peripheral eosinophilia and CMR was performed immediately at first day of visit before cardiac biopsy. CMR showed diffuse subepicardial high T2 signals and diffuse late gadolinium enhancement in LV. Steroid therapy was immediately initiated and patient's symptom was rapidly improved. Endomyocardial biopsy at hospital day 3 reported multifocal mild infiltration of eosinophils and lymphocytes. The patient was finally confirmed as acute eosinophilic myopericarditis. This presentation emphasizes on the role of CMR which enables early non-invasive diagnosis of AEM and visualize the extent of the myocarditis.
Acute eosinophilic myopericarditis (AEM) is a rare disorder of unknown etiology, which is characterized by diffuse or focal myocardial inflammation with eosinophilic infiltration. It frequently results in cardiogenic shock and a fatal clinical course if diagnosis is delayed. Because it usually dramatically responds to steroid therapy, early diagnosis and treatment possibly reduce the mortality and morbidity in AEM (1, 2). Here we report the case of early diagnosis of AEM by cardiovascular magnetic resonance (CMR) before pathologic report. The patient was successfully treated at early stage of AEM without sequelae.
A 35-yr-old woman presented to the emergency department on April 30, 2010 with flu-like illness and malaise for 3 days, followed by pleuritic chest pain and shortness of breath. She had a history of gastric band surgery due to morbid obesity. She had no medical history of allergy or regular medications. There was no travel or recent animal exposure history.
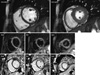
On arrival, her heart rate was 80 beats/min and a blood pressure of 90/70 mmHg. Cardiovascular examination showed no jugular venous distension, gallops or murmurs. Laboratory values reveals: white blood cells 12,030/µL (normal range, 4,500-10,000/µL); absolute eosinophil count 1,624/µL (normal range, 50-350/µL); creatinine kinase 72 U/L (normal range, 24-173 U/L); creatinine kinase MB 3.96 U/L (normal range, < 5 U/L); troponin 1.343 ng/mL (normal range, < 0.78 ng/mL); N-terminal proBNP: 3,465 pg/mL (normal range, < 150 pg/mL); erythrocyte sedimentation rate 3 mm/hr (normal range, < 27 mm/hr); and C-reactive protein 0.71 mg/dL (normal range, < 0.3 mg/dL). The electrocardiogram showed sinus rhythm with nonspecific ST-segment and T-wave abnormalities and the chest radiography was normal. The transthoracic echocardiography showed borderline left ventricular (LV) systolic dysfunction with slightly increased LV wall thickness (LV interventricular septum, 11 mm; LV posterior wall, 11 mm at end diastole) and small amount of pericardial effusion (Fig. 1). CMR was performed with the protocol of cine image, T2 weighted image, and delayed gadolinium-enhanced imaging with phase-sensitive inversion recovery (PSIR) technique after injection of 0.15 mM/kg Gadovist (gadobutrol; Bayer Schering Pharma, Berlin, Germany) using an 1.5-T scanner (Magnetom Avanto, Syngo MR B15 version; Siemens Medical Solutions, Erlangen, Germany). Cine image showed borderline LV systolic function (LV EF = 51%) with slightly increased LV wall thickness (11 mm at diastole), same as echocardiography. elevation of T2 signal intensity was found in whole LV myocardium and basal posterior side of pericardium and diffuse delayed hyper-enhancement especially in the LV septum, anterior and anteriolateral wall and moderate pericardial effusion (Fig. 2). The presumptive diagnosis based on the clinical feature and CMR findings was AEM. Steroid therapy was initiated with oral prednisone at 1 mg/kg/day. After being started on steroid therapy, her peripheral eosinophilia resolved and her symptoms improved from hospital day 1. Although, clinical course and CMR showed high probability of AEM, we performed endomyocardial biopsy 2 days later after initiation of steroid therapy; because until now, it is a golden standard for diagnosis of myocarditis. Histopathology revealed multifocal mild infiltration of mixed eosinophils and lymphocytes (Fig. 3), suggesting a diagnosis of AEM. She was finally confirmed AEM.
A short term follow up echocardiogram 3 days after initiation of steroid therapy showed improvement in systolic function, normalization of LV wall thickness and decreased pericardial effusion. After 2 months, follow-up CMR showed absence of pericardial effusion, and high signal intensity in T2 image, and no abnormal LGE in parallel with clinical improvement (Fig. 4). Ejection fraction has also improved to completely normal range (63%).
Early diagnosis and treatment is important to reduce the mortality and morbidity in AEM. Pathologic diagnosis by endomyocardial biopsy is the standard diagnostic method. However, it is invasive procedure and takes several hours to days to confirm the results. Furthermore, it is not a very sensitive technique because the infiltrates in AEM are often focal (estimated sensitivity at 50%) (3). Recently, CMR is frequently used as a supportive diagnostic tool in suspected acute myocarditis in clinical decision making. CMR can visualize the inflammation and tissue edema in entire myocardium, which is ideal for a disease with patchy involvement of myocardium such as myocarditis, and the course of AEM during treatment (4).
CMR using T1-weighted early gadolinium enhancement, T2-weighted edema imaging, and LGE imaging using appropriate pulse sequences were reported as sensitive methods to detect changes in tissue composition. Abdel-Aty et al. (5) reported that higher T1-weighted early gadolinium enhancement had a sensitivity of 80% and specificity of 73% in diagnosis of the patients with myocarditis. They also found that increased T2 signal intensity had a sensitivity of 84% and specificity of 74% and LGE in subepicardial myocardium had a sensitivity of 44% and specificity of 100%. When T2-weighted CMR is combined with gadolinium enhancement imaging, it had a high diagnostic accuracy in patients with suspected acute myocarditis (6). LGE pattern of myocarditis is characterized by diffuse enhancement of myocardium. According to the clinical course, diffuse enhancement changes into patch involvement, and remains scar in some patients. In myocarditis patients, cardiac enzyme usually elevated and differential diagnosis of acute myocardial infarction is crucial for management of patients. LGE in acute myocardial infarction is characterized by subendocardial involvement of LGE according to the coronary artery disease, and easily discriminates myocarditis from acute myocardial infarction with high sensitivity and specificity. In case of cardiac amyloidosis, diffuse LGE pattern may be found, however T2 signal usually does not increase (7).
Our patient with peripheral eosinophilia, immediately underwent CMR before cardiac biopsy suggested findings of acute myopericarditis. Early high dose corticosteroids therapy leads to dramatic improvement before the pathologic diagnosis of AEM. After 2 months of steroid therapy, follow-up CMR revealed no residual delayed hyperenhancement suggesting favorable clinical outcome without any residual pathology in myocardium. This presentation emphasizes on the role of CMR which enables early non-invasive diagnosis of AEM and visualize the extent of the myocarditis.
Figures and Tables
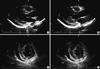 | Fig. 1Transthoracic echocardiography showed borderline LV systolic function with increased LV wall thickness and a small amount of pericardial effusion. (A) parasternal long axis view of LV at end diastole. (B) parasternal long axis view of LV at end systole. (C) parasternal short axis view of LV at end diastole. (D) parasternal short axis view of LV at end systole. |
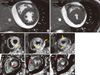 | Fig. 2CMR at initial diagnosis. (A, B) Cine CMR showed borderline LV systolic function (LV EF = 51%), slightly increased LV wall thickness (11 mm at end-diastole) and moderate amount of pericardial effusion. (C-E) T2 signals were increased in LV myocardium and pericardium (yellow arrows). (F-H) There was diffuse delayed enhancement in myocardium of LV, especially in septal and apical wall. Pericardium at posterior side was slightly enhanced at DHE image. |
 | Fig. 3Pathology of endomyocardial biopsy (hematoxylin and eosin staining) shows multifocal mild infiltration of mixed eosinophils and lymphocytes with suspicious myocyte damage, which is compatible with eosinophilic myocarditis. (A) under low magnification (× 400), (B) under high magnification (× 1,000). |
References
1. Al Ali AM, Straatman LP, Allard MF, Ignaszewski AP. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol. 2006. 22:1233–1237.
2. Sohn IS, Park JC, Chung JH, Kim KH, Ahn Y, Jeong MH, Cho JG. A case of acute eosinophilic myopericarditis presenting with cardiogenic shock and normal peripheral eosinophil count. Korean J Intern Med. 2006. 21:136–140.
3. Burke AP, Saenger J, Mullick F, Virmani R. Hypersensitivity myocarditis. Arch Pathol Lab Med. 1991. 115:764–769.
4. Debl K, Djavidani B, Buchner S, Poschenrieder F, Heinicke N, Feuerbach S, Riegger G, Luchner A. Time course of eosinophilic myocarditis visualized by CMR. J Cardiovasc Magn Reson. 2008. 10:21.
5. Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005. 45:1815–1822.
6. Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991. 68:1089–1091.
7. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005. 26:1461–1474.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download