Abstract
Since 1987, dura mater graft-associated iatrogenic Creutzfeldt-Jakob disease (dCJD) has been reported in many countries. We report the first case of dCJD in Korea. A 54-yr-old woman, who underwent resection of the meningioma in the left frontal region and received a dura mater graft 23 yr ago presented with dysesthesia followed by psychiatric symptoms and ataxia. Her neurological symptoms rapidly progressed to such an extent that she exhibited myoclonus, dementia, and pyramidal and extrapyramidal signs within 8 weeks. The 14-3-3 protein was detected in her cerebrospinal fluid; however, an electroencephalogram did not reveal characteristic positive sharp wave complexes. Diffusion-weighted magnetic resonance images, obtained serially over 64 days, revealed the rapid progression of areas of high signal intensity in the caudate nucleus and cingulate gyrus to widespread areas of high signal intensity in the cortex and basal ganglia. Pathological examination of brain biopsy specimens confirmed the presence of spongiform changes and deposition of prion protein in the neurons and neuropils.
Creutzfeldt-Jakob disease (CJD) is a fatal, transmissible, neurodegenerative disorder that causes spongiform changes in the brain. CJD has 4 clinical forms: sporadic, genetic, iatrogenic, and variant. Sporadic and genetic CJD appear to occur in people from all countries and ethnic groups. Furthermore, to date, more than 400 cases of iatrogenic CJD have been reported in 20 countries (1). No studies have reported cases of iatrogenic CJD in Korea. Iatrogenic CJD results from the inadvertent transmission of CJD during the course of medical or surgical treatment. The sources of infection reported in iatrogenic CJD were cadaveric dura mater grafts (2), human cadaveric pituitary hormone (3), corneal transplants (4), and neurosurgical equipment (5, 6). Since the first identification of dura mater graft-associated CJD (dCJD) in 1987, almost 200 cases of dCJD have been identified worldwide (1). Here, we report the first case of dCJD in a patient in Korea, who had received a dura mater graft with Lyodura® 23 yr ago.
A 54-yr-old woman was referred to a tertiary referral hospital because of ataxia and myoclonus in June 2010. She was diagnosed with meningioma in the left frontal area, and the meningioma was excised in 1987. One month before visiting the tertiary referral hospital, she first began experiencing general weakness and dysesthesia on the left side of her face and in the toes of her right foot. The findings of the brain magnetic resonance imaging (MRI) (T1-weighted, T2-weighted, and FLAIR images without diffusion-weighted images [DWIs]) performed at a neurology outpatient clinic were not remarkable, except for encephalomalacic changes that occurredas a sequela of brain surgery. Subsequently, her neurological symptoms and signs progressed further to such an extent that she developed dysarthria and could not stand up without assistance. Immediately after admission to the tertiary referral hospital, she exhibited phobia, marked mood fluctuations, and insomnia. The first DWIs in the second MRI, performed 24 days after the first MRI, revealed slightly increased signal intensity in the cingulate gyrus and caudate nuclei (Fig. 1). Discrete decreases in signal intensity were observed in the corresponding area in analog to digital converter aliasing (ADC) maps (data not shown), indicating reduced diffusivity. Five days after admission, she could not walk alone because of ataxia. Ten days after admission, she exhibited multifocal generalized myoclonus without seizures. During the next 2 weeks, her motor symptoms worsened further to such an extent that she developed anarthria and severe dysphagia. The higher cortical function test could not be performed because she could not speak. However, she could understand simple commands and was partially communicable. She complained of fear due to visual hallucinations and diplopia. The third follow-up MRI, performed 1 month after the second one, revealed areas of increased signal intensity in the right and left basal ganglia, insular cortex, primary motor cortex, and right and left medial frontal cortex (Fig. 1). One and a half months after the initial admission, she was confined to bed with frequent myoclonus. Her mental status had changed to a drowsy or stuporous state, and she slept during most of the day. An electroencephalogram (EEG) revealed moderate diffuse slowing of background activity without periodic sharp wave complexes. The 14-3-3 protein was detected in her cerebrospinal fluid. There was no mutation in PRNP, and the genotype of codon 129 was methionine homozygote. She was tentatively diagnosed with CJD and referred to our CJD surveillance center for confirmative diagnosis. A careful review of her past medical records revealed that Lyodura® had been used to repair her dura in 1987. The fourth MRI, performed 1 month after the third MRI, revealed a high signal intensity lesion in both basal ganglia, both insulae, and the right and left frontotemporoparietal cortices (Fig. 1). A brain needle biopsy was performed at the right frontal region. Hematoxylin and eosin staining of biopsy specimens revealed neuronal loss and astrocytosis with spongiform changes (Fig. 2). Immunostaining with a monoclonal antibody (1C5) against prion protein revealed deposition of prion protein in the peri- and intraneuronal areas. We did not observe florid plaques in the biopsy specimens. The patient was discharged from the hospital to receive supporting treatment. She died after an overall disease course of 3 months.
To date, nearly 200 cases of iatrogenic dCJD have been reported worldwide (1). In this patient, CJD, confirmed by examination of brain biopsy specimens, was related to a dura mater graft. She underwent a dura mater graft performed using Lyodura®, a brand of dura mater linked to an ongoing international outbreak of most of the reported cases of dCJD (1, 2). In this patient, the latent period between Lyodura® grafting and the onset of CJD was 23 yr, which is within the range observed in the reported cases of dCJD (16 months-31 yr) (1, 7-11).
More than 50% of the cases of dCJD have been identified in Japan. dCJD constitutes 6.2% of all CJD cases reported in Japan, and 135 cases of dCJD have been reported since November 2009 (12). However, this is the first case of iatrogenic dCJD in Korea. The estimated risk for dCJD after exposure to human dura mater was reported to be 1 per 500 to 2000, depending on the study population and the year when the dura mater graft was performed (8, 13, 14). However, an accurate estimation of the use of human dura mater grafts in Korea is not available.
Studies have suggested that ataxia and psychiatric symptoms or signs are among the earliest symptoms of dCJD and are followed by visual symptoms, dementia, myoclonus, and extrapyramidal or pyramidal signs (7-9, 11). The clinical course of our patient followed this pattern. The MRI findings in our case resembled those observed in sporadic CJD in other studies (7-9, 11). We were able to review serial MR images obtained 4 times over 64 days when the patient was transferred to our center. While she was symptomatic, the areas of high signal intensity on DWIs progressed rapidly from suspicious changes in the cingulate gyrus and caudate nucleus to extensive cortical and basal ganglia involvement. Given the long latency of dCJD, the occurrence of these MRI changes within a short period in this case is remarkable.
Although needle biopsy specimens may not represent the entire brain, we believe that our patient presented with neuropathologically non-plaque typed CJD (10). In a study of non-plaque type dCJD reported in Japan, all cases exhibited positive sharp wave complexes on EEG, unlike in our case (9). This might be explained by differences in the time between symptom onset and EEG investigation. In a Japanese non-plaque typedCJD series, a case with positive sharp wave complexes was detected 5 months after symptom onset.However, the last EEG followup for our patient was 2.5 months after symptom onset.
In conclusion, we report the first case of dura mater-graft associated iatrogenic CJD in Korea. We observed a long incubation period in our patient, similar to that observed in other countries, and thus, iatrogenic CJD cases due to dura mater transplants still can be expected in the future.
Figures and Tables
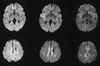
Fig. 1
Serial DWIs taken during the course of dCJD. The first DWI (left column), taken almost 6 weeks after her first neurological symptoms, revealedslightly increased signal intensity in the cingulate gyrus and the caudate nucleus. In the second DWI (middle column), taken 10 weeks after her first neurological symptoms, high signal intensity in both insulae, the caudate nucleus, and the medial frontal cortical region became obvious. In the third DWI (right column), taken 12 weeks after her first neurological symptoms, extensive high signal intensity was observed in the both basal ganglia and the cortex.
References
1. Brown P, Brandel JP, Preece M, Sato T. Iatrogenic Creutzfeldt-Jakob disease: the waning of an era. Neurology. 2006. 67:389–393.
2. Thadani V, Penar PL, Partington J, Kalb R, Janssen R, Schonberger LB, Rabkin CS, Prichard JW. Creutzfeldt-Jakob disease probably acquired from a cadaveric dura mater graft. Case report. J Neurosurg. 1988. 69:766–769.
3. Tintner R, Brown P, Hedley-Whyte ET, Rappaport EB, Piccardo CP, Gajdusek DC. Neuropathologic verification of Creutzfeldt-Jakob disease in the exhumed American recipient of human pituitary growth hormone: epidemiologic and pathogenetic implications. Neurology. 1986. 36:932–936.
4. Hogan RN, Cavanagh HD. Transplantation of corneal tissue from donors with diseases of the central nervous system. Cornea. 1995. 14:547–553.
5. Simpson DA, Masters CL, Ohlrich G, Purdie G, Stuart G, Tannenberg AE. Iatrogenic Creutzfeldt-Jakob disease and its neurosurgical implications. J Clin Neurosci. 1996. 3:118–123.
6. Bryce EA, Dorovoni-Zis K, Trudeau D, Sinclair M, Roberts FJ. Creutzfeldt-Jakob disease: management of accidental contamination of neurosurgical instruments, pathology equipment, and solutions. Infect Control Hosp Epidemiol. 2000. 21:247–248.
7. Heath CA, Barker RA, Esmonde TF, Harvey P, Roberts R, Trend P, Head MW, Smith C, Bell JE, Ironside JW, Will RG, Knight RS. Dura mater-associated Creutzfeldt-Jakob disease: experience from surveillance in the UK. J Neurol Neurosurg Psychiatry. 2006. 77:880–882.
8. Brooke FJ, Boyd A, Klug GM, Masters CL, Collins SJ. Lyodura use and the risk of iatrogenic Creutzfeldt-Jakob disease in Australia. Med J Aust. 2004. 180:177–181.
9. Noguchi-Shinohara M, Hamaguchi T, Kitamoto T, Sato T, Nakamura Y, Mizusawa H, Yamada M. Clinical features and diagnosis of dura mater graft associated Creutzfeldt Jakob disease. Neurology. 2007. 69:360–367.
10. Yamada M, Noguchi-Shinohara M, Hamaguchi T, Nozaki I, Kitamoto T, Sato T, Nakamura Y, Mizusawa H. Dura mater graft-associated Creutzfeldt-Jakob disease in Japan: clinicopathological and molecular characterization of the two distinct subtypes. Neuropathology. 2009. 29:609–618.
11. Meissner B, Kallenberg K, Sanchez-Juan P, Ramljak S, Krasnianski A, Heinemann U, Eigenbrod S, Gelpi E, Barsic B, Kretzschmar HA, Schulz-Schaeffer WJ, Knauth M, Zerr I. MRI and clinical syndrome in dura mater-related Creutzfeldt-Jakob disease. J Neurol. 2009. 256:355–363.
12. Yamada M, Nozaki I, Hamaguchi T, Noguchi-Shinohara M, Kitamoto T, Nakamura Y, Sato T, Mizusawa H. Prion disease surveillance in Japan: analysis of 1,241 patients. Rinsho Shinkeigaku. 2009. 49:939–942.
13. Centers for Disease Control and Prevention (CDC). Update: Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts: Japan, 1979-2003. MMWR Morb Mortal Wkly Rep. 2003. 52:1179–1181.
14. Nakamura Y, Yanagawa H, Hoshi K, Yoshino H, Urata J, Sato T. Incidence rate of Creutzfeldt-Jakob disease in Japan. Int J Epidemiol. 1999. 28:130–134.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download