Abstract
Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a recently described mesenchymal tumor of the stomach. We report the first case of PAMT in Korea. A 52-yr-old man underwent esophagogastroduodenoscopy due to dyspepsia for 2 yr. There was a submucosal mass with small mucosal ulceration in the gastric antrum. The tumor measured 3.5 × 2.3 cm in size and showed multinodular plexiform growth pattern of bland-looking spindle cells separated by an abundant myxoid or fibromyxoid matrix rich in small thin-walled blood vessels. The tumor cells were negative for CD117 (c-KIT), CD34 and S-100 protein, but diffusely positive for smooth muscle actin consistent with predominant myofibroblastic differentiation. The patient is doing well without recurrence or metastasis for 5 months after surgery. Although there have been limited follow-up data, PAMT is regarded as a benign gastric neoplasm with histological and immunohistochemical charateristics distinguished from gastrointestinal stromal tumor and other mesenchymal tumors of the stomach.
The most common gastrointestinal mesenchymal tumor is a gastrointestinal stromal tumor (GIST) (1). Plexiform angiomyxoid myofibroblastic tumor (PAMT) is a recently described mesenchymal tumor of the stomach, characterized by plexiform nodular growth of bland spindle cells in myxoid or fibromyxoid stroma (2). Although myxoid tumors of the stomach have been described as gastric myxoma and fibromyxoma in the older literature before the concept of PAMT (3), we could find 22 tumors similar to our case in the literature under the diagnostic name of PAMT, plexiform angiomyxoid tumor or plexiform fibromyxoma since Takahashi et al. (2) first described in 2007 (3-10). Here we would like to report a new case of PAMT of gastric antrum, including preoperative endoscopic biopsy findings and a review of the literature. To the best of our knowledge it must be the first PAMT case in Korea.
A 52-yr-old man visited the hospital due to gastric mass found at local clinic on January 11, 2011. He has no specific symptom except dyspepsia for 2 yr. On esophagogastroduodenoscopic examination, about 5 cm-sized movable mass with mucosal ulceration was detected in the gastric antrum (Fig. 1A). An endoscopic biopsy was done under the clinical impression of GIST. Pathologic examination of the mass showed an ill-defined myxoid lesion composed of loosely scattered bland spindle cells and small thin-walled blood vessels in the gastric mucosa (Fig. 1B). The spindle cells were positive for smooth muscle actin (SMA) (1A4, 1:40, Dakocytomation, Carpinteria, CA, USA), but negative for CD117 (polyclonal, 1:250, Dakocytomation), CD34 (QBEND10, 1:30, Dakocytomation) and S-100 protein (polyclonal, 1:150, Zymed, San Francisco, CA, USA) immunostains (Fig. 1C, D). Histological findings favored a benign mesenchymal tumor, but no precise diagnosis was made. Endoscopic ultrasonogram revealed a 2.3 × 2.1 cm-sized hypoechoic lesion in muscle proper layer, but abdominal CT had no remarkable finding. The patient underwent laparoscopic wedge resection of the gastric mass on February 28, 2011.
A gross examination showed a multinodular glistening mass of 3.5 × 2.3 cm in size, with an umbilicated ulcer in the overlying mucosa (Fig. 2A). The mass was mainly located in the submucosal layer and poorly demarcated from the muscularis propria (Fig. 2B). Microscopically, the tumor showed a multinodular plexform growth pattern dissecting the wall (Fig. 3A). The tumor nodules consisted of bland-looking spindle cells separated by an abundant myxoid or fibromyxoid matrix (Fig. 3B, C). Fascicular or palisading arrangement of the tumor cells was also noted (Fig. 3D). Numerous small thin-walled blood vessels were observed in the myxoid stroma (Fig. 3E). In some nodules, the collagenous stroma was densely hyalinized. The spindle tumor cells had no significant nuclear atypia and mitotic activity. Immunohistochemically, they were diffusely positive for SMA and focally positive for desmin (ZC18, 1:60, Zymed) (Fig. 3F), but negative for CD117, CD34 and S-100 protein as described in the endoscopic biopsy. The patient received supportive care without any specific medication during hospitalization and had no recurrence or metastasis in the follow-up for 5 months after the surgery.
PAMT of the stomach is a very rare neoplasm. Miettinen et al. (3) estimated the incidence of PAMT as less than 1/150 compared with that of gastric GIST. It occurs over a wide range of ages (3, 8). When we analyzed all 23 PAMTs including this case, based on the previous reports (2-10), age of the patients ranged from 7 to 75 yr (mean, 42 yr) and there were 12 males and 11 females. All of the tumors occurred in the gastric antrum, therefore they could be excised by partial or distal gastrectomy. More than half of the cases (13/23) showed erosion or ulceration of gastric mucosa, and gastrointestinal bleeding was the most common symptom. The tumor size ranged from 1.9 to 15 cm (mean, 6.1 cm). Immunohistochemically, all cases were positive for muscle specific actin or SMA and negative for CD117 (KIT), CD34 and S-100 protein. There was focal positivity for caldesmon, desmin, or CD10 in some cases (3, 8).
Takahashi et al. (2) named two cases of tumor, which have not been described previously, as PAMT to designate a peculiar histology (plexiform growth pattern, a myxoid stroma rich in small vessels) and cell nature (myofibroblastic origin). They confirmed myofibroblastic nature of tumor cells by immunohistochemical and ultrastructural analyses. PAMT is mainly composed of tumor cells with myofibroblastic phenotype, but may also contain tumor cells with fibroblastic or smooth muscle characteristics. Miettinen et al. (3) reported the presence of tumor cells with fibroblastic traits (focal CD10-positivity) and Yoshida et al. (6) reported that a minority of tumor cells possess smooth muscle differentiation (focal positivity for desmin and caldesmon, blunted end nuclei and long eosinophilic cytoplasmic extensions). They proposed terms such as "plexiform fibromyxoma" and "plexiform angiomyxoid tumor" respectively to describe tumors similar to PAMT. Our case showed a focal immunoreactivity for desmin consistent with smooth muscle differentiation, in addition to diffuse positivity for SMA consistent with myofibroblastic differentiation. Because the majority of cases have represented predominant myofibroblastic nature of tumor cells as in our case, we think that PAMT is an appropriate diagnostic term to cover histogenesis and histology. However, the WHO classification of tumors of the digestive system accepted "plexiform fibromyxoma" as a diagnostic term instead of PAMT (1). Plexiform fibromyxoma, proposed by Miettinen et al. (3), was based on histological appearance of tumors and stromal spectrum from extensively myxoid to collagenous. However, Sing et al. (9) suggested that plexiform fibromyxoma is distinguished from PAMT by immunohistochemical and clinicopathologic features. Mean tumor size of plexiform fibromyxoma was larger than that of PAMT and vascular invasion and extragastric extension of the tumor were typically observed in plexiform fibromyxomas, but not in PAMT. In addition, desmin and caldesmon showed no reactivity in plexiform fibromyxoma, but they were focally positive in PAMT. Based on these findings they considered PAMT and plexiform fibromyxoma as related entities with variable fibroblastic-myofibroblastic differentiation, with plexiform fibromyxoma occupying the dominant fibroblastic end of spectrum, while PAMT is fully differentiated myofibroblastic tumor.
PAMT should be separated from other myxoid and fibromyxoid tumors of the stomach including myxoid variant of GIST, myxoid leiomyoma, plexiform neurofibroma and inflammatory fibroid polyp. GIST does not show the distinctive plexiform intramural growth pattern and are typically positive for CD117 or DOG1. Myxoid leiomyoma is positive for SMA, desmin and caldesmon and plexiform neurofibroma is positive for S-100 protein. Inflammatory fibroid polyp is usually small submucosal lesion composed of epithelioid to spindled fibroblasts and inflammatory cells.
Although the true biologic potential of PAMT remains unknown due to its rarity and limited follow-up data, the short term prognosis has been good with no recurrence or metastasis reported. Histological features such as bland nuclear morphology, low proliferative index, no necrosis suggest that PAMT is a benign tumor.
Here we report the first case of PAMT in Korea. PAMT has a distinctive histological and immunohistochemical features distinguished from GIST and other mesenchymal tumors. When myxoid spindle cell lesion is observed in endoscopic biopsy, PAMT should be included in differential diagnosis.
Figures and Tables
Fig. 1
Endoscopic examination of the tumor. (A) The stomach shows a submucosal mass with small mucosal ulceration in the antrum. (B) The biopsied mucosa shows an ill-defined myxoid lesion with loosely scattered spindle cells (H&E, × 40). The spindle tumor cells were positive for smooth muscle actin (C) and negative for CD117 (D) immunostains (× 200).
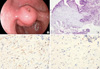
Fig. 2
Gross examination of the excised specimen. (A) There is a protruding multinodular glistening mass, mainly located in the submucosa. (B) Cut surface showed myxoid nature and unclear margin of the tumor.

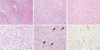
Fig. 3
Microscopic examination of the tumor. (A) The tumor showed plexiform growth pattern dissecting the muscularis propria (H&E, × 10). The bland-looking spindle tumor cells were separated by abundant myxoid (B) or fibromyxoid stroma (C) (H&E, × 100). (D) Palisading arrangement of tumor cells was noted (H&E, × 200). (E) There were numerous thin-walled vessels in the myxoid stroma (H&E, × 200). (F) The tumor cells were focally positive for desmin immunostain (× 200).
References
1. Miettinen M, Fletcher CD, Kindblom LG, Tsui WM. Bosman FT, Carneiro F, Hruban R, Theise ND, editors. Mesenchymal tumours of the stomach. WHO classification of tumours of the digestive system. 2010. Lyon: IARC;74–79.
2. Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2007. 31:724–728.
3. Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009. 33:1624–1632.
4. Rau TT, Hartmann A, Dietmaier W, Schmitz J, Hohenberger W, Hofstaedter F, Katenkamp K. Plexiform angiomyxoid myofibroblastic tumour: differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin Pathol. 2008. 61:1136–1137.
5. Galant C, Rousseau E, Ho Minh Duc DK, Pauwels P. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2008. 32:1910.
6. Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol. 2008. 32:1910–1912.
7. Pailoor J, Mun KS, Chen CT, Pillay B. Plexiform angiomyxoid myofibroblastic tumour of the stomach. Pathology. 2009. 41:698–699.
8. Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol. 2010. 16:2835–2840.
9. Sing Y, Subayan S, Mqadi B, Ramdial PK, Reddy J, Moodley MS, Bux S. Gastric plexiform angiomyxoid myofibroblastic tumor. Pathol Int. 2010. 60:621–625.
10. Tan CY, Santos LD, Biankin A. Plexiform angiomyxoid myofibroblastic tumor of the stomach: a case report. Pathology. 2010. 42:581–583.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download