Abstract
Natural isoflavones and flavones are important dietary factors for prostate cancer prevention. We investigated the molecular mechanism of these compounds (genistein, biochanin-A and apigenin) in PC-3 (hormone-independent/p53 mutant type) and LNCaP (hormone-dependent/p53 wild type) prostate cancer cells. A cell growth rate and apoptotic activities were analyzed in different concentrations and exposure time to evaluate the antitumor activities of genistein, biochanin-A and apigenin. The real time PCR and Western blot analysis were performed to investigate whether the molecular mechanism of these compounds are involving the p21 and PLK-1 pathway. Apoptosis of prostate cancer cells was associated with p21 up-regulation and PLK-1 suppression. Exposure of genistein, biochanin-A and apigenin on LNCaP and PC-3 prostate cancer cells resulted in same pattern of cell cycle arrest and apoptosis. The inhibition effect for cell proliferation was slightly greater in LNCaP than PC-3 cells. In conclusion, flavonoids treatment induces up-regulation of p21 expression, and p21 inhibits transcription of PLK-1, which promotes apoptosis of cancer cells.
Prostate cancer is the second commonly occurring cancer in western countries as well as in Asian countries, and the incidence rates are rapidly growing (1). The conventional approaches, such as radical prostatectomy, radiotherapy and hormonal therapy, have not been managed effectively for this cancer (2).
Among risk factors of prostate cancer, dietary habit is significantly associated with reduced risks for prostate cancer (3). Isoflavones and flavones are recommended to reduce prostate cancer (4). The major isoflavones are genistein and biochanin-A. Genistein has anti-angiogenic effects and blocks the uncontrolled cancer cell growth, and the cytotoxic activity is based on tyrosine kinase inhibition and DNA topoisomerase II inhibition (5). The other important isoflavone is biochanin-A, a methoxylated isoflavone in red clover, which induces delay of the S phase into the G2/M phase progression (6), and is a powerful agonist of the human aryl-hydrocarbon (ArH) receptor (7). Plant flavone apigenin has the effect of cell growth inhibition, anti-inflammatory, anticancer and free-radical scavenging properties (8). Apigenin promotes cell cycle arrest and apoptosis, through induced G2/M phase cell cycle arrest by suppression of cyclin B (9, 10).
Recent studies have shown that genistein, biochanin-A and apigenin inhibit the growth of prostate cancer cells via the promotion of cell cycle arrest and apoptosis, but the exact cell-cycle mechanism remains unknown. Therefore, we focused on the cell division regulators. One of considering key regulator is polo-like kinases (PLKs), particularly, PLK-1 activity (11, 12). PLK-1 is a potent regulator for multiple cell cycle functions, including activation of Cdc2, mitotic entry, bipolar spindle formation, centrosome maturation, and cytokinesis (13). PLK-1 depletion results in induction of apoptosis, so the inhibition of PLK-1 activity is a promising approach for the anticancer therapy (14). The down-regulation of PLK-1 expression occurs through a transcriptional repression mechanism (15), with this reason, p21 was investigated as another key factor. p21 was reported as a negative PLK-1 transcription regulator of cell cycle progression at G1 phase (16).
Until now, PLK-1 and p21 have been shown to have anti-cancer activity individually, but there is no report on the simultaneous detection in prostate cancer cells. Therefore, we investigated the apoptotic effects of genistein, biochanin-A and apigenin on the prostate cancer cell lines through p21 mediated PLK-1 transcriptional regulation mechanism.
Two human prostate carcinoma cell lines differing in androgen association and p53 status were used in this study. LNCaP (wild-type for p53, androgen-dependent) and PC-3 (mutant-type for p53, androgen-independent) were purchased from American Type Culture Collection (Rockville, MD, USA) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. All of the cells were maintained at standard cell culture conditions (37℃, 5% CO2 in a humidified incubator).
Genistein, biochanin-A and apigenin (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO) (final concentration 0.1% in medium). Cells (2 × 103) were plated in flat-bottomed 96-well plates and treated with 0, 10, 50 and 100 µM of genistein and biochanin-A, and 0, 10, 20, 40, and 80 µM of apigenin, respectively. After incubation for 72 hr at 37℃, 20 µL of methyl thiazolyl tetrazolium (MTT) (Sigma-Aldrich) solution was added to each well and then the absorbance was measured at 490 nm using the VERSAmax microplate reader (Molecular Device, Sunnyvale, CA, USA).
Cells (2 × 106) were cultured in 100 mm tissue culture dishes for 24 hr, and treated with genistein (100 µM), biochanin-A (100 µM) and apigenin (40 µM). After 36 hr, the cells were washed with phosphate buffered saline (PBS), detached by treatment with Trypsin/EDTA, and then centrifuged at 1,000 g for 5 min. The collected cells were incubated in the dark with Annexin V-FITC and annexin binding buffer (1 × ) at 24℃ for 15 min and then centrifuged at 1,000 g for 5 min. Annexin binding buffer (1 × ) and propidium iodide staining solution were added to the cells and analyzed using a FACS Calibur instrument (BD Biosciences, San Jose, CA, USA).
RNA isolation was performed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacture's protocol. Reverted first strand cDNA Synthesis Kit (Fermentas Life Sciences, Glen Burnie, MD, USA) was used for cDNA synthesis. Two micrograms of cDNA was used as a template for PCR reaction. The resulting cDNA samples were amplified by quantitative PCR in the presence of SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA). The following primers were used: GAPDH-sense 5'-TGG GCT ACA CTG AGC ACC AG-3' and -antisense 5'-GGG TGT CGC TGT TGA AGT CA-3', Plk1-sense 5'-ATA GAG CGT GAC GGC ACT GAG T-3' and -antisense 5'-TGC TCG CTC ATG TAA TTG CG-3', and p21-sense 5'-CAA AGG CCC GCT CTA CAT CTT-3' and -antisense 5'-AGG AAC CTC TCA TTC AAC CGC-3'. The cycling conditions were as follows: initial denaturation (10 min at 95℃), followed by 40 cycles of denaturation (15 sec at 95℃), annealing (30 sec at 60℃) and elongation (30 sec at 72℃) and a final extension (10 min at 72℃). The comparative Ct method was used to calculate the relative changes in gene expression in 7500 Fast Real-time PCR System (Applied Biosystems). The relative changes of gene expression were calculated using the following formula: Fold change in gene expression, 2-ΔΔCt = 2-{ΔCt (treated samples)-ΔCt (untreated control)}, where ΔCt = Ct (detected genes)-Ct (GAPDH) and Ct represents threshold cycle number.
Cells (2 × 106) were cultured in 100 mm tissue culture dishes for 24 hrs, and were treated with genistein (100 µM), biochanin-A (60 µM) and apigenin (40 µM). After 48 hr, the cells were washed with PBS, detached by treatment with Trypsin/EDTA, and then centrifuged at 1,000 g for 5 min. The cells were lysed in RIPA lysis buffer (150 mM NaCl, 1% NP-40, 0.5% Doc, 0.1% SDS, 50 mM Tris [pH 8.0]). The lysed cells were centrifuged at 12,000 rpm for 10 min. Protein determination was performed by using the BCA Protein Assay Reagent kit (Pierce, Rockford, IL, USA). Cell lysates (40 µg) were electrophoresed in 8% SDS polyacrylamide gels and transferred onto nitrocellulose membranes. After blotting in 5% non-fat dry milk in Tween 20 Tris-buffered saline (TTBS), the membranes were incubated in a blocking solution (25 mM tris [pH 7.5], 150 mM NaCl, 0.1% Tween 20, 5% skim milk) at room temperature for 1 hr and then probed with 1:200 dilution of mouse monoclonal antibody for p21 and PLK-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃ for 12 hr. The membranes were then washed with TTBS and incubated with 1:1,000 dilution of goat anti-mouse polyvalent IgG-HRP (Santa Cruz Biotechnology) at room temperature for 1 hr. The membranes were washed in TTBS, and bands were visualized on X-ray film using Immobilion Western Kit (Millipore, Bedford, MA, USA).
LNCaP and PC-3 cells were treated with different doses of genistein, biochanin-A or apigenin for 3 days, and cell viability was assessed by the MTT assay. Genistein, biochanin-A and apigenin induced decrease of cell viability of both cell lines in a dose-dependent manner. Genistein and biochanin-A showed significant inhibition of cell proliferation at the dose of 50 and 100 µM. Apigenin showed growth inhibition at 40 and 80 µM, significantly. More considerable reduction of cell viability occurred in LNCaP cells than in PC-3 cells treated with genistein and biochanin-A (Fig. 1). Isoflavones (genistein and biochanin-A) and flavone (apigenin) showed similar pattern for the reduction of cell viability.
Flow cytometric analysis of Annexin V-FITC showed that treatment with genistein, biochanin-A and apigenin induced about 3-fold increase (21.3%, 20.5% and 23.5%, respectively) in early apoptotic fraction (Q4) of LNCaP cells, compared to control group (7.4%). Late apoptotic fraction (Q2) of LNCaP cells showed about 1.8-fold to 3.2-fold increase in apoptosis (7.9% for genistein, 6.9% for biochanin-A and 12.2% for apigenin) compared to control group (3.8%). In the analysis of combination of early and late apoptotic fraction, apoptosis was increased about 2.4- to 3.1-fold (11.2% for control, 29.2% for genistein, 27.4% for biochanin-A and 35.7% for apigenin) (P < 0.05) (Fig. 2A).
In PC-3 cells, treatment with genistein, biochanin-A and apigenin induced about 2.5-fold to 4-fold increase in apoptosis (8.6%, 11.0% and 13.8%, respectively) in early apoptotic fraction, compared to control group (3.4%). The late apoptotic fraction showed about 1.6-fold to 8.7-fold increase in apoptosis (6.3%, 14.9% and 2.8%, respectively) compared to control group (1.7%) (Fig. 2B). In the analysis of combination of early and late apoptotic fraction, apoptosis was increased about 2.6- to 5-fold (5.1% for control, 14.9% for genistein, 25.9% for biochanin-A and 16.7% for apigenin) (P < 0.05) (Fig. 2B).
Inhibition of cell proliferation is through an arrest in cell-cycle and up-regulation of apoptosis. We investigated effect on cell cycle by evaluating the transcriptional expression of PLK-1 gene in prostate cancer cells. On LNCaP cell line, minimum 10 µM of genistein, 100 µM of biochanin-A and 40 µM of apigenin treatment showed p21 up-regulation, and 50 µM of genistein, 50 µM of biochanin-A and 40 µM of apigenin treatment showed PLK-1 inhibition, significantly (P < 0.05).
In the PC-3 cell line, minimum 100 µM of genistein, 50 µM of biochanin-A and 40 µM of apigenin treatment showed p21 up-regulation, and 100 µM of genistein, 50 µM of biochanin-A and 40 µM of apigenin treatment showed PLK-1 inhibition significantly, (P < 0.05) (Fig. 3).
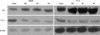
The effect of genistein, biochanin-A and apigenin on the protein expression of this signaling pathway was confirmed using western blot. The results indicated that genistein, biochanin-A and apigenin treatment for 48 hrs induced increase the expression of p21 and decrease of PLK-1 protein (Fig. 4).
Our experiment demonstrated that genistein, biochanin-A and apigenin affect inhibition of prostate cancer cell proliferation after induction of apoptosis through promoter element p21 for transcriptional inhibition of PLK-1.
The cell proliferation rate, flow cytometry, real-time PCR and western blot analysis showed that treatment of the flavonoids induced similar regulation mechanism for LNCaP and PC-3 cells. Genistein, biochanin-A and apigenin treatment caused a remarkable decrease of PLK-1 expression levels. PLK-1 is a well-recognized key regulator of the mitotic progression, notably, a close correlation observed between PLK-1 expression and carcinogenesis including prostate cancer (17). PLK-1 induced tumorigenesis is based on centrosome abnormality, particularly centrosome amplification defects. Centrosome duplication and maturation was regulated by PLK-1 at late S phase to prophase. PLK-1 overexpression increases the centrosome size and number, which leads to multipolar spindles and unequal segregation of chromosomes (18). While, PLK-1 inhibition results in inactivation of cyclin-dependent kinase 1 (Cdc2)/cyclin B 1-mediated mitotic arrest, which causes cell apoptosis (12).
PLK-1 transcription level is controlled by regulator, located at promoter region. PLK-1 expression level is low at the G1→S transition, increases at the S phase, and maximum expression showed at G2→M stage (12). One of a negative regulator of PLK-1 transcription is p53, tumor suppressor gene, and the detailed connection of PLK-1 and p53 has been evaluated (19). However, other negative regulators for PLK-1 transcription are not studied intensively yet.
Our experiment focused on a correlation of p21 expression and PLK-1 inhibition, because p21 controls PLK-1 transcription as a negative regulator in cancer cells (20). Using LNCaP and PC-3 cell lines, cell cycle analyses indicated that the genistein, biochanin-A and apigenin treatment showed an obvious delayed mitosis and this phenotype was associated with the elevation of p21. According to reports, elevation of p21 after PLK-1 inhibition might be a general phenomenon in normal and cancer cells (21, 22). In normal cells, stabilization of p21 induces PLK-1 depletion, which causes the elevation of p53. In cancer cells (p53-defective), PLK-1 depletion reduced Mdm2 expression, a negative regulator of p21 protein stability (21). Thus, anti-cancer effects of genistein, biochanin-A and apigenin are associated with down-regulation of PLK-1 and up-regulation of p21 mechanism.
Our result is the first study for overexpression of p21 and suppression of PLK-1 on prostate cancer cells through mechanism for p21 induced inhibition of PLK-1 transcription. Even though, there have been a few studies about the effect of flavonoids on the expression of PLK-1 and p21, all of them studied individual effect of p21 and PLK-1. Ismail et al. (23) reported that genistein induced PLK-1 down-regulation-mediated apoptosis and G2/M arrest on neuroblastoma cells. Reagan-Shaw and Ahmad (17) reported that blocking PLK-1 expression via siRNA induced apoptosis and G2/M arrest in human prostate cancer cells. Zhao et al. (24) showed that p21 was upregulated by genistein in both p53-expressing LNCaP and p53-null PC-3 cells. Apigenin exposure increased p53 protein expression, which correlated with an increase in the levels of its transcriptional target p21 on prostate cancer cells (25).
This study proved that one of anti-prostate cancer mechanisms by genistein, biochanin-A and apigenin through increase of p21 pathway resulted in decreased PLK-1 expression as a consequence of apoptotic cell death. Therefore, it is concluded that genistein, biochanin-A and apigenin treatment relates to the combined p21 and PLK-1 mechanism, which could be a potentially attractive strategy in prostate cancer cell therapy.
Figures and Tables
Fig. 1
Dose dependent inhibition of cell proliferation by genistein (GEN), biochanin-A (BIO) and apigenin (Api) on LNCaP and PC-3 prostate cancer cells. The data represent mean ± SD of six independent experiments.

Fig. 2
Induction of apoptosis by genistein, biochanin-A and apigenin on LNCaP and PC-3 prostate cancer cells.

Fig. 3
Up-regulation of p21 and inhibition of PLK-1 gene expression by real-time PCR analysis treating genistein, biochanin-A and apigenin on LNCaP and PC-3 prostate cancer cells.

Fig. 4
p21 mediated PLK-1 protein regulation by western blot analysis treating genistein, biochanin-A and apigenin on LNCaP and PC-3 prostate cancer cells. Genistein (GEN, 100 µM), biochanin-A (BIO, 60 µM) and apigenin (API, 40 µM) were treated for 48 hr. Control cells (Ctrl) were treated with 0.1% DMSO.
AUTHOR SUMMARY
Apoptotic Effects of Genistein, Biochanin-A and Apigenin on LNCaP and PC-3 Cells by p21 through Transcriptional Inhibition of Polo-like Kinase-1
Young Jin Seo, Bum Soo Kim, So Young Chun, Yoon Kyu Park, Ku Seong Kang and Tae Gyun Kwon
Genistein, biochanin-A and apigenin induce cell growth inhibition by increasing apoptosis in prostate cancer cells. These apoptotic mechanisms involved enhancement of p21 expression and inhibition of PLK-1 expression. Genistein, apigenin and biochanin- A may have antitumor activity and may be useful as an adjuvant to standard therapy for prostate tumors.
References
1. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003. 53:5–26.
2. Trojan L, Kiknavelidze K, Knoll T, Alken P, Michel MS. Prostate cancer therapy: standard management, new options and experimental approaches. Anticancer Res. 2005. 25:551–561.
3. Kucuk O. Chemoprevention of prostate cancer. Cancer Metastasis Rev. 2002. 21:111–124.
4. Boué SM, Carter-Wientjes CH, Shih BY, Cleveland TE. Identification of flavone aglycones and glycosides in soybean pods by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003. 991:61–68.
5. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001. 24:351–356.
6. Ying C, Hsu JT, Hung HC, Lin DH, Chen LF, Wang LK. Growth and cell cycle regulation by isoflavones in human breast carcinoma cells. Reprod Nutr Dev. 2002. 42:55–64.
7. Medjakovic S, Jungbauer A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J Steroid Biochem Mol Biol. 2008. 108:171–177.
8. Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001. 39:139–147.
9. Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH Jr, Iwamura T, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006. 5:76.
10. Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000. 28:102–110.
11. Dai W, Cogswell JP. Polo-like kinases and the microtubule organization center: targets for cancer therapies. Prog Cell Cycle Res. 2003. 5:327–334.
12. Ahmad N. Polo-like kinase (Plk) 1: a novel target for the treatment of prostate cancer. FASEB J. 2004. 18:5–7.
13. Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008. 24:475–499.
14. Jalili A, Moser A, Pashenkov M, Wagner C, Pathria G, Borgdorff V, Gschaider M, Stingl G, Ramaswamy S, Wagner SN. Polo-like kinase 1 is a potential therapeutic target in human melanoma. J Invest Dermatol. 2011. 131:1886–1895.
15. McKenzie L, King S, Marcar L, Nicol S, Dias SS, Schumm K, Robertson P, Bourdon JC, Perkins N, Fuller-Pace F, Meek DW. p53-dependent repression of polo-like kinase-1 (PLK1). Cell Cycle. 2010. 9:4200–4212.
16. Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009. 10:265–275.
17. Reagan-Shaw S, Ahmad N. Silencing of polo-like kinase (Plk) 1 via siRNA causes induction of apoptosis and impairment of mitosis machinery in human prostate cancer cells: implications for the treatment of prostate cancer. FASEB J. 2005. 19:611–613.
18. Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007. 17:60–65.
19. Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, Fukuzawa M, Nakagawara A. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004. 279:25549–25561.
20. Salvatore G, Nappi TC, Salerno P, Jiang Y, Garbi C, Ugolini C, Miccoli P, Basolo F, Castellone MD, Cirafici AM, Melillo RM, Fusco A, Bittner ML, Santoro M. A cell proliferation and chromosomal instability signature in anaplastic thyroid carcinoma. Cancer Res. 2007. 67:10148–10158.
21. Kreis NN, Sommer K, Sanhaji M, Kramer A, Matthess Y, Kaufmann M, Strebhardt K, Yuan J. Long-term downregulation of Polo-like kinase 1 increases the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). Cell Cycle. 2009. 8:460–472.
22. Lei M, Erikson RL. Plk1 depletion in nontransformed diploid cells activates the DNA-damage checkpoint. Oncogene. 2008. 27:3935–3943.
23. Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007. 575:12–20.
24. Zhao R, Xiang N, Domann FE, Zhong W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer. 2009. 61:397–407.
25. Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008. 44:1833–1845.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download