Abstract
KITENIN (KAI1 C-terminal interacting tetraspanin) promotes invasion and metastasis in mouse colon cancer models. In the present study, we evaluated the effects of KITENIN knockdown by intravenous administration of short hairpin RNAs (shRNAs) in an orthotopic mouse colon cancer model, simulating a primary or adjuvant treatment setting. We established orthotopic models for colon cancer using BALB/c mice and firefly luciferase-expressing CT-26 (CT26/Fluc) cells. Tumor progression and response to therapy were monitored by bioluminescence imaging (BLI). In the primary therapy model, treatment with KITENIN shRNA substantially delayed tumor growth (P = 0.028) and reduced the incidence of hepatic metastasis (P = 0.046). In the adjuvant therapy model, KITENIN shRNA significantly reduced the extent of tumor recurrence (P = 0.044). Mice treated with KITENIN shRNA showed a better survival tendency than the control mice (P = 0.074). Our results suggest that shRNA targeting KITENIN has the potential to be an effective tool for the treatment of colon cancer in both adjuvant and metastatic setting.
KAI1 encodes a transmembrane glycoprotein of the tetraspanin family and was initially described as a prostate-specific metastasis suppressor (1-3). Down-regulation or loss of KAI1 expression occurs in advanced stages of many types of human cancer and is associated with poor prognosis, leading to the proposal that KAI1 might be a metastasis suppressor (4-12).
Previously, we cloned KITENIN (KAI1 C-terminal interacting tetraspanin), a member of the tetraspanin protein family that interacts specifically with the C-terminal cytoplasmic domain of KAI1 (13). In contrast to KAI1, KITENIN increases migration and invasiveness of colon cancer cells and knockdown of KITENIN inhibits tumor metastasis in a mouse colon cancer model (13-15). Recently, we reported the signaling mechanism of KITENIN at cellular level. KITENIN serves as a scaffolding molecule that simultaneously recruits both Dishevelled (Dvl) and protein kinase Cδ (PKCδ) through the membrane-spanning C-terminal region to form a complex that stimulates extracellular signal-regulated kinase (ERK)/activating protein-1 (AP-1) via a PKCδ component but also organizes the actin filament via a Dvl component (15). KITENIN knockdown distorts the actin arrangement and decreases AP-1 target genes such as MMP-1, MMP-3 and CD44, thereby suppresses tumor cell invasion and metastasis (15). These results suggest that the knockdown of KITENIN could be useful for the treatment of colon cancer together with other chemotherapeutic agents.
The current study was designed to investigate the systemic effects of KITENIN knockdown on a clinically relevant orthotopic mouse colon cancer model, simulating a primary treatment or adjuvant setting.
To silence the expression of KITENIN, five regions of mouse KITENIN were targeted for small interfering RNAs (siRNAs). Two of the five regions were shown to specifically reduce the expression of mouse KITENIN (KITENIN S2, 5'-GCGCTATCTGGGCCTTACC-3'; KITENIN S5, 5'-AGAAGCGGAGAGCAAGACT-3'). To generate siRNAs, equimolar amounts of complementary sense and antisense strands were each mixed and annealed in a 50 µL reaction at 90℃ for 4 min, 70℃ for 10 min, 60℃ for 30 min, 37℃ for 20 min, and 25℃ for 10 min. The pSUPER vector (OligoEngine, Seattle, WA, USA) was digested with BglII and HindIII and the annealed oligos were ligated into the vector to express short hairpin RNAs (shRNAs), which are processed in vivo as siRNA-like molecules capable of carrying out gene-specific silencing. The sequences of the inserts in the two resulting shRNA expression plasmids, KITENIN shRNA2 and KITENIN shRNA5, were determined and no errors were found.
BALB/c mice (5-7 weeks of age) were obtained from Jungang Lab Animal, Inc. (Seoul, Korea) and kept in our laboratory animal facility, maintained at 23℃ ± 2℃ with a relative humidity of 50% ± 20% and a 12-hour light/dark cycle. All methods used in this study (2010-3) were approved by the institutional animal care and use committee at the Chonnam National University Medical School Research Institution and conformed to NIH guidelines (NIH publication No. 86-23, revised 1985). The murine BALB/c-derived colon carcinoma cell lines, CT-26 and firefly luciferase-expressing CT-26 (CT26/Fluc) cells were kindly provided by Dr. Min JJ (Chonnam National University, Korea).
The IVIS200 imaging system (Xenogen, Alameda, CA, USA), which included an optical charge-coupled device (CCD) camera mounted on a light-tight specimen chamber, was used for data acquisition and analysis. Firefly D-luciferin potassium salt, the Fluc substrate, was diluted to 3 mg/100 µL in PBS before use. The mice were intraperitoneally injected with 100 µL of this D-luciferin solution. Each mouse was placed in a specimen chamber mounted with a CCD camera, which had been cooled to -105℃ and whose field of view (FOV) was set to 26 cm above the sample shelf. Light emitted by luciferase in the mice was then measured. Gray scale photographic images and bioluminescent color images were superimposed using LIVINGIMAGE V. 2.12 software (Xenogen) and IGOR image analysis software. Bioluminescence signals are expressed in units of photons per second per centimeter squared per steradian (p/s/cm2/sr). Background signal intensity in vivo was in the range of 1-3 × 105 photons/sec.
Subcutaneous xenografts were established by injecting firefly luciferase-expressing CT-26 (CT26/Fluc) cells (1 × 106 cells per mouse) into the right flank of BALB/c mice, and growth was monitored regularly. Tumors were selected for orthotopic implantation at an average size of 1 cm3 and the tissue was cut into 1.5 mm3 pieces. Implantation was performed according to the method described by Pocard and colleagues with some modifications (16). In brief, the cecum was exteriorized through a small midline laparotomy and a piece of tumor tissue derived from CT26/Fluc cells was sutured to the cecal surface with a single Maxon 7/0 suture, leaving the tumor tissue buried in a 'pouch' consisting of a double cecal wall on each side. After implantation, the abdominal wall was closed in two layers with Dexon 5/0. Ten days after cecal implantation of the colon tumor, the groups of mice were randomly assigned to receive one of the following two treatments (n = 6 per group): (a) tail vein injections of an empty pSUPER vector with FuGene 6 transfection reagent (Roche, Seoul, Korea) at intervals of 4 days, and (b) tail vein injections of KITEININ shRNA with FuGene 6 at intervals of 4 days. The KITENIN shRNA was a mixture of KITENIN shRNA2 and KITENIN shRNA5, administered at doses of 100 µg in a volume of 100 µL per injection for each mouse as previously described (14). To facilitate delivery of the vectors or shRNA in vivo, the Fugene 6 transfection reagent was applied according to the manufacturer's protocol. Each mouse was analyzed by optical imaging at intervals of 4 days after tumor implantation.
The adjuvant therapy model was based on the orthotopic mouse colon cancer model described earlier. When the total flux of the implanted tumor was determined by the in vivo imaging system (IVIS) to have exceeded 5 × 106, a cecectomy (tumor resection) was performed under general anesthesia. The abdomen was prepared for sterile surgery, and a median incision was made. The colon was exposed after careful dissection and hemostasis of peritoneal adhesion. The cecum was fully resected using a surgical clip as reported by Kuo et al. (17). The bowel was replaced in the abdominal cavity, and the wound was closed in two layers. We selected mice with bioluminescence signal intensities below 3 × 105 photons/sec when imaged one day after tumor resection, and randomly assigned them to three groups: surgery only, CPT-11 (Irinotecan hydrochloride, Pfizer, New York, NY, USA) treatment, or KITENIN shRNA treatment group. CPT-11 is an effective chemotherapeutic agent for colorectal cancer (18, 19) and we used CPT-11 as a reference tool to examine the efficacy of KITENIN shRNA. At the time of randomization, each mouse expressed only a background bioluminescence signal and differences in signal intensities among the three groups were not significant (P = 0.806, Table 1). In retrospective analysis of the preoperative signals in the three randomized groups, there were no differences in signal intensities among them (P = 0.767, Table 1). A total dose of 100 µg of KITENIN shRNA was given by intravenous (i.v.) injection on postoperative days 2, 5, and 8, and CPT-11 (40 mg/kg) was given by intraperitoneal (i.p.) injection on postoperative days 2, 5, and 8. Each group was composed of 8 mice. One mouse from the control group, two from the CPT-11 treatment group, and two from the KITENIN shRNA treatment group died from postoperative complications within five days after randomizations. Seven mice from the control group and six mice each from the CPT-11 and KITENIN shRNA treatment groups were finally analyzed. Tumor recurrence in mice was monitored weekly using in vivo bioluminescence imaging (BLI). All surviving animals were sacrificed on the ninth postoperative week.
At 4 weeks after tumor implantation, the mice from each group in primary therapy model were sacrificed. Specimens from the tumor bed, peritumoral lymphovascular structure, and liver were fixed with 4% formaldehyde in PBS, dehydrated with ethanol, embedded in paraffin blocks, sectioned in 4 µm increments and stained with hematoxylin-eosin and KITENIN.
Differences in bioluminescence signals were analyzed by a Mann-Whitney test. Differences in the incidence of mucosal invasion, peritumoral lymphovascular invasion, and liver metastasis were analyzed by two-way Fisher's exact and chi-squared tests. The difference in tumor volume was analyzed by a Student's two-tailed t-test. The survival curves were calculated according to the Kaplan-Meier Method and the differences in survival were analyzed using the log-rank test. In all cases, P < 0.05 was considered to be significant.
Since observation that KITENIN shRNA markedly suppresses KITENIN expression ex vivo and in vivo was reported previously (14), this study evaluated the effects of the intravenous administration of KITENIN shRNA on tumor burden, invasion, and metastasis using a clinically relevant orthotopic mouse colon cancer model. Fig. 1A shows a typical example of the assessment of signal intensity using in vivo BLI. All mice in both groups showed tumor progression as evidenced by increasing bioluminescence signal intensities, but the administration of KITENIN shRNA substantially delayed tumor growth in comparison with the control vector-treated group. At day 20, 24, and 28 after tumor implantation, the KITENIN shRNA treatment group showed significant signal inhibition compared to the control group (P < 0.05; Fig. 1B).
When the mice were sacrificed for autopsy on day 28 after orthotopic implantation, metastatic mass of the liver was often observed in the control group (Fig. 2A). In the mice treated with KITENIN shRNA, the tumor growth tended to be confined to the region of the cecum where the tumor had been implanted and exhibited slower growth. Treatment with KITENIN shRNA resulted in a 64% reduction in tumor volume relative to vehicle control mice (P = 0.028; n = 6 per group, Table 2). Histological findings of the primary tumor, peritumoral lymphovascular structure, and liver were assessed in tissue sections for the evaluation of tumor invasion and metastasis. Mucosal invasion was detected in two of the six KITENIN shRNA-treated mice and in five of the six control mice (P = 0.079; Table 2, Fig. 2B-D). Tumor embolus in the lymphovascular structure was detected in one of the six KITENIN shRNA-treated mice and in three of the six control mice (P = 0.221; Table 2, Fig. 2E, F). Metastatic nodules in the liver were observed in three of the six control mice and not observed in KITENIN shRNA-treated mice (P = 0.046; Table 2, Fig. 2G, H).
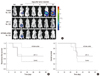
The data above confirmed that KITENIN shRNA treatment is effective for the treatment of established mouse colon cancer. Next, we hypothesized that adjuvant KITENIN shRNA treatment would reduce recurrence and prolong survival following surgical resection of the colon tumor mass in mice. To test this hypothesis, we established an animal model simulating an adjuvant treatment setting. Tumor recurrence in mice was monitored weekly using in vivo BLI (Fig. 3A). Recurrence-free survival data according to treatment group was given in Fig. 3B. The KITENIN shRNA treatment group achieved superior results compared to the control group (P = 0.044). There were no differences in recurrence-free survival between the CPT-11 treatment and KITENIN shRNA treatment groups or between the CPT-11 treatment and control groups. The recurrence rates in the control group, CPT-11 treatment group, and KITENIN shRNA treatment group were 71.4% (5 of 7), 50.0% (3 of 6), and 16.7% (1 of 6), respectively. To examine whether the tumor suppression effect of KITENIN shRNA treatment could yield a survival benefit, survival within the three groups was calculated by Kaplan-Meier analysis. At the end of the experimental period, there were 2, 3, and 5 survivors in the control, CPT-11, and KITENIN shRNA-treated mouse groups, respectively. Mice treated with KITENIN shRNA showed a tendency toward increased survival compared to the control mice (P = 0.074; Fig. 3C).
The primary aim of the present study was to investigate the effects of KITENIN knockdown by intravenous administration of KITENIN shRNA after primary tumor establishment, or postoperatively as an adjuvant treatment in an orthotopic mouse colon cancer model. Suitable animal models that mimic the clinical situation are essential to develop new therapeutic strategies. Fidler (20, 21) and Morikawa et al. (22) reported that orthotopic implantation was necessary to induce metastasis of colon cancer in mice, i.e. a higher rate of liver metastasis by colon cancer was observed when tumors were implanted on the cecum compared with subcutaneous implantation. Using BLI, tumors in the same animal can be sequentially visualized and the tumor volume can be quantified, as reported for various tumor models (23-29). Previous studies have validated the use of BLI in a murine orthotopic colorectal cancer metastasis model and reported strong correlations between the bioluminescent signal intensity and tumor mass (24, 30). The bioluminescent signal intensity is depth-dependent, resulting in a higher signal for superficial lesions than for deeper lesions. The other major limitation of BLI is that the planar nature of the images impedes accurate 3-dimensional localization of the signal (30). Nevertheless, BLI is a sensitive and powerful tool for high-throughput longitudinal monitoring of tumor load in small animals and allows for the implementation of more advanced orthotopic tumor models in therapy intervention studies. In the present study, orthotopic mouse models for colon cancer were established successfully by using firefly luciferase-expressing CT-26 murine adenocarcinoma cells. In the primary therapy model, administration of KITENIN shRNA substantially delayed growth of the established tumor and reduced the incidence of liver metastasis in comparison with the control vector-injected group. These data reconfirm our previous report that KITENIN suppression by shRNA inhibits tumor progression and liver metastasis in a subcutaneous tumor model (14). In the adjuvant therapy model, KITENIN shRNA treatment achieved superior results compared to the control group in terms of recurrence-free survival rate (Fig. 3B). Mice treated with KITENIN shRNA demonstrated a tendency toward increased survival compared to control mice (Fig. 3C). At the time of randomization, there were no differences in bioluminescence signal intensities among the three groups (P = 0.806). This allows us to exclude biases in residual disease among the three groups, which supports the reliability of the estimation of the effectiveness of the treatments in an adjuvant setting. In our adjuvant experimental model, all mice with recurrent tumor died during the follow-up period, but not of the mice without tumor recurrence. The recurrence rate (death rate) was lower in the KITENIN shRNA treatment group (16.7%) compared to the CPT-11 treatment group (50.0%), although the difference was not statistically significant (P = 0.195). These results suggest that gene therapy with shRNA targeting KITENIN could be as useful as chemotherapy for the treatment of colon cancer.
We recently examined the expression of KITENIN in paraffin-embedded samples of primary colon tumors, adjacent normal colonic mucosa, metastatic mass of the liver and lymph node from the patients with colon cancer by immunohistochemical staining (15). Compared with the expression in the adjacent normal mucosa, KITENIN was highly expressed in the tumor. There was a positive correlation between the expression level of KITENIN and advanced stages of human colon cancer. Also, KITENIN expression in metastatic lymph nodes and liver masses was comparable with that of colon tumors in stage IV colon cancer. These results suggested that gene therapy using an anti-KITENIN strategy might be effective for human colon cancer.
In summary, this study demonstrated that systemic administration of KITENIN shRNA inhibits tumor progression, distant metastasis, and recurrence in orthotopic mouse colon cancer models. These results suggest that shRNA targeting KITENIN has the potential to be an effective tool for the treatment of colon cancer in both adjuvant and metastatic setting.
Figures and Tables
Fig. 1
Quantitative photon counting analysis of the progression of orthotopically implanted tumors. (A) Detection of the progression process in control and KITENIN shRNA treatment groups. (B) Quantitative analysis of the progression process in control (○) and KITENIN shRNA treatment groups (▲) (n = 6). Points, mean of six mice per group; bars, SE.
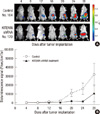
Fig. 2
Macroscopic and microscopic appearance of tumor growth after orthotopic implantation. (A) Necropsy pictures are shown. A red circle outlines the implanted tumor and a blue circle outlines the hepatic nodule. (B) H&E section of a tumor-bearing cecum. (C, D) Higher magnifications of the black box in B showing tumor invasion into the mucosa (control group) or tumor growth not penetrating the mucosa and submucosa (KITENIN shRNA-treated group). M, mucosa; SM, submucosa; Tm, tumor. Asterisks in D indicate invasion into the adjacent colonic crypt. (E, F) Peritumoral lymphovascular tumor embolus (TE). (G, H) Metastatic nodule of the liver. Black arrows indicate a subcapsular tumor nodule in the liver.
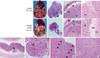
Fig. 3
Tumor recurrence was monitored weekly using in vivo bioluminescence imaging (BLI) and recurrence-free survival was estimated. (A) Detection of recurrence after tumor resection by in vivo BLI. PI#1, post-implantation 1; BOP, before operation. (B, C) Recurrence-free survival and overall survival according to treatment in the adjuvant treatment model. Time scale relates to days after tumor resection.
AUTHOR SUMMARY
Intravenous KITENIN shRNA Injection Suppresses Hepatic Metastasis and Recurrence of Colon Cancer in an Orthotopic Mouse Model
Jun-Eul Hwang, Hyun-Jeong Shim, Young-Kyu Park, Sang-Hee Cho, Woo-Kyun Bae, Dae-Eun Kim, Kyung-Keun Kim and Ik-Joo Chung
Despite the development of chemothepeutic agents colon cancer, the prognosis of recurred or metastatic colon cancer has been poor. KITENIN promotes invasion and metastasis in colon cancer. We found that KITENIN knockdown by intravenous administration of shRNAs substantially delayed the tumor growth and reduced the extent of tumor recurrence in a mouse colon cancer model. Our results suggest that shRNA targeting KITENIN can be the potentially effective method for the treatment of colon cancer.
References
1. Rinker-Schaeffer CW, Hawkins AL, Ru N, Dong J, Stoica G, Griffin CA, Ichikawa T, Barrett JC, Isaacs JT. Differential suppression of mammary and prostate cancer metastasis by human chromosomes 17 and 11. Cancer Res. 1994. 54:6249–6256.
2. Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995. 268:884–886.
3. Dong JT, Suzuki H, Pin SS, Bova GS, Schalken JA, Isaacs WB, Barrett JC, Isaacs JT. Down-regulation of the KAI1 metastasis gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996. 56:4387–4390.
4. Adachi M, Taki T, Ieki Y, Huang CI, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996. 56:1751–1755.
5. Schindl M, Birner P, Breitenecker G, Oberhuber G. Downregulation of KAI1 metastasis suppressor protein is associated with a dismal prognosis in epithelial ovarian cancer. Gynecol Oncol. 2001. 83:244–248.
6. Su JS, Arima K, Hasegawa M, Franco OE, Umeda Y, Yanagawa M, Sugimura Y, Kawamura J. Decreased expression of KAI1 metastasis suppressor gene is a recurrence predictor in primary pTa and pT1 urothelial bladder carcinoma. Int J Urol. 2004. 11:74–82.
7. Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998. 153:973–983.
8. Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003. 200:39–46.
9. Kim JH, Kim MA, Lee HS, Kim WH. Comparative analysis of protein expressions in primary and metastatic gastric carcinomas. Hum Pathol. 2009. 40:314–322.
10. Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett. 2006. 240:183–194.
11. Jackson P, Marreiros A, Russell PJ. KAI1 tetraspanin and metastasis suppressor. Int J Biochem Cell Biol. 2005. 37:530–534.
12. Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal. 2009. 21:196–211.
13. Lee JH, Park SR, Chay KO, Seo YW, Kook H, Ahn KY, Kim YJ, Kim KK. KAI1 COOH-terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res. 2004. 64:4235–4243.
14. Lee JH, Cho ES, Kim MY, Seo YW, Kho DH, Chung IJ, Kook H, Kim NS, Ahn KY, Kim KK. Suppression of progression and metastasis of established colon tumors in mice by intravenous delivery of short interfering RNA targeting KITENIN, a metastasis-enhancing protein. Cancer Res. 2005. 65:8993–9003.
15. Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Lee JH, Seo YW, Ahn KY, Chung IJ, Kim KK. KITENIN recruits Dishevelled/PKC delta to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut. 2009. 58:509–519.
16. Pocard M, Muleris M, Hamelin R, Salmon RJ, Dutrillaux B, Poupon MF. Growth dependency of human colon cancer xenograft on organ environment is related with their original clinical stage. Anticancer Res. 1998. 18:2743–2747.
17. Kuo TH, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, Kitajima M, Hoffman RM. Early resection of primary orthotopically-growing human colon tumor in nude mouse prevents liver metastasis: further evidence for patient-like hematogenous metastatic route. Anticancer Res. 1993. 13:293–297.
18. Cunningham D, Pyrhönen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998. 352:1413–1418.
19. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000. 355:1041–1047.
20. Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990. 50:6130–6138.
21. Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995. 87:1588–1592.
22. Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988. 48:6863–6871.
23. Dickson PV, Hamner B, Ng CY, Hall MM, Zhou J, Hargrove PW, McCarville MB, Davidoff AM. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007. 42:1172–1179.
24. Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, Kasahara N. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007. 67:5345–5353.
25. Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, Ross BD. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000. 2:491–495.
26. Sadikot RT, Blackwell TS. Bioluminescence imaging. Proc Am Thorac Soc. 2005. 2:537–540. 511–512.
27. Wang Y, Sun Z, Peng J, Zhan L. Bioluminescent imaging of hepatocellular carcinoma in live mice. Biotechnol Lett. 2007. 29:1665–1670.
28. Yanagihara K, Takigahira M, Takeshita F, Komatsu T, Nishio K, Hasegawa F, Ochiya T. A photon counting technique for quantitatively evaluating progression of peritoneal tumor dissemination. Cancer Res. 2006. 66:7532–7539.
29. Jones-Bolin S, Zhao H, Hunter K, Klein-Szanto A, Ruggeri B. The effects of the oral, pan-VEGF-R kinase inhibitor CEP-7055 and chemotherapy in orthotopic models of glioblastoma and colon carcinoma in mice. Mol Cancer Ther. 2006. 5:1744–1753.
30. Smakman N, Martens A, Kranenburg O, Borel Rinkes IH. Validation of bioluminescence imaging of colorectal liver metastases in the mouse. J Surg Res. 2004. 122:225–230.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download