Abstract
This study aimed to compare the clinical presentations of Aeromonas hydrophila, A. veronii biovar sobria and A. caviae monomicrobial bacteremia by a retrospective method at three hospitals in Taiwan during an 8-yr period. There were 87 patients with A. hydrophila bacteremia, 45 with A. veronii biovar sobria bacteremia and 22 with A. caviae bacteremia. Compared with A. hydrophila and A. veronii biovar sobria bacteremia, A. caviae bacteremia was more healthcare-associated (45 vs 30 and 16%; P = 0.031). The patients with A. caviae bacteremias were less likely to have liver cirrhosis (27 vs 62 and 64%; P = 0.007) and severe complications such as shock (9 vs 40 and 47%; P = 0.009) and thrombocytopenia (45 vs 67 and 87%; P = 0.002). The APACHE II score was the most important risk factor of Aeromonas bacteremia-associated mortalities. The APACHE II scores of A. caviae bacteremias were lower than A. hydrophila bacteremia and A. veronii biovar sobria bacteremia (7 vs 14 and 16 points; P = 0.002). In conclusion, the clinical presentation of A. caviae bacteremia was much different from A. hydrophila and A. veronii biovar sobria bacteremia. The severity and mortality of A. caviae bacteremia were lower than A. hydrophila or A. veronii biovar sobria bacteremia.
Aeromonas is a kind of oxidase-producing gram-negative rods and belongs to the family Aeromonadaceae. It is widely distributed globally in aquatic environments and associated with a variety of human infections, including gastroenteritis, soft tissue infection, septicemia, hepatobiliary tract infections, and occasionally pleuropulmonary infections, indwelling-device related infections, meningitis, peritonitis, and hemolytic uremic syndrome (1). Although this pathogen could infect healthy persons, most infections were found in immunocompromised hosts, especially those with liver cirrhosis and malignancies (1-4). The possible pathogenesis of Aeromonas infection is complex and multifactorial. The possible portals of entry for Aeromonas bacteremia were considered as gastrointestinal tracts and skin lesions (1-5). After the adhesion to the epithelial cells of the intestine, this pathogen produces many potential virulence factors to destroy epithelial barrier and impair immune cells, including exoenzymes, cytotoxic and cytotonic enterotoxins (6, 7).
Among 21 Aeromonas species differentiated on the basis of DNA-DNA hybridization, Aeromonas caviae, Aeromonas veronii biovar sobria and Aeromonas hydrophila are most associated with human infections and amount for > 85% of all clinical isolates (2-5). They have different biochemical properties (8, 9) and antimicrobial susceptibilities (10, 11). In addition, different Aeromonas species produce different virulence factors (12). However, it is unknown whether different Aeromonas species contribute different clinical presentations. Here, we conducted a retrospective study to compare clinical presentations of the bacteremias caused by different Aeromonas species.
This is a retrospective study, in which the patients diagnosed with monomicrobial Aeromonas bacteremia were admitted at Buddhist Tzu Chi General Hospital, Buddhist Dalin Tzu Chi General Hospital and Buddhist Taipei Tzu Chi General Hospital (Taiwan) from January 2001 to November 2008. Buddhist Tzu Chi General Hospital is a 700-bed tertiary referral medical center locating in Eastern Taiwan with special units for bone marrow and organ transplantation, burn care and intensive care. Buddhist Dalin Tzu Chi General Hospital and Buddhist Taipei Tzu Chi General Hospital are 900-bed regional teaching hospitals locating in Southern Taiwan and Northern Taiwan. The demographic, clinical and laboratory information were retrieved from the medical charts of the included patients for analysis.
Aeromonas bacteremia was defined as growth of an Aeromonas sp. from a blood culture of a patient with sepsis. Bacteremia was considered healthcare-associated if an Aeromonas isolate was obtained from blood sampled after more than 72 hr of hospitalization in a patient who had been asymptomatic for infection upon admission, or from a patient who had received antineoplastic chemotherapy in the preceding 2 weeks after drawing blood for culture, regardless of symptomatology at admission. Aeromonas-involved polymicrobial bacteremia defined as simultaneous growth of an Aeromonas sp. and other microbe(s) from a blood culture of a patient with sepsis were excluded from this study. Death was considered to be attributable to Aeromonas bacteremia if, during the same hospital stay, death occurred within 7 days after a positive blood culture for Aeromonas bacteremia without other cause for death, death occurred in the presence of clinical evidence of persistent sepsis, or the cause of death as recorded on the death certificate was Aeromonas bacteremia. Survivor from Aeromonas bacteremia was defined as if the patient was discharged alive or an improvement of bacteremia-associated symptoms occurred in the absence of recurrence within 30 days during the same hospital stay.
According to the Sepsis-related Organ Failure Score criteria, the diagnosis of respiratory failure is based on the ratio of arterial oxygen tension (PaO2) to fractional inspired oxygen (FiO2) < 200 mmHg. Disease severity was assessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) score within 72 hr after the occurrence of symptoms associated with Aeromonas bacteremia. Acid-suppressant therapy was defined as use of proton pump inhibitors or histamine H2 blockers for more than 7 days within 4 weeks before onset of the symptoms associated with Aeromonas bacteremia.
Blood samples were tested daily for microbial growth by the BACTEC 9240 (BD, Diagnostic Instrument Systems, Spark, MD, USA). Gram-negative bacilli from blood culture bottles were identified as Aeromonas species by positive oxidase reaction, no growth on thiosulfate-citrate-bile-sucrose agar, growth on MacConkey agar, and resistance to the vibriostatic compound O/129. Biochemical profiles with the Vitek II system (bioMérieux, Lyon, France), BD-Phoenix system (BD Diagnostic Instrument Systems) or API-20NE system (bioMérieux) were utilized for identification of Aeromonas species. Additional tests for API-20NE system included hydrolysis of esculin and gas production from glucose fermentation. Additional tests for Vitek II system or BD-Phoenix system included hydrolysis of esculin, Voges-Proskauer reaction, acid production from sucrose fermentation, ornithine decarboxylase, acid from arabinose fermentation, and arginine dihydrolase production.
In vitro antimicrobial susceptibilities of Aeromonas isolates were tested using the Kirby-Bauer disk-diffusion method, or automated methods (Vitek II system or the BD-Phoenix system). Antibiotics selected for testing included ampicillin, amikacin, cefazolin, gentamicin, cefmetazole, ceftriaxone, cefuroxime, ciprofloxacin, imipenem, flomoxef, cefpirome, ceftazidime, sulfamethoxazole/trimethoprim, aztreonam, ticarcillin/clavulanic acid, and piperacillin/tazobactam. The breakpoint concentrations for interpretation were in accordance with Clinical and Laboratory Standards Institute (13).
SPSS v. 11.5 for MS Windows software (SPSS, Chicago, IL, USA) software was used for statistical analyses. Pearson's chi-square test was used to examine nominal data, and one-way ANOVA was used for continuous data. All tests were two-sided and a P value of ≤ 0.05 was considered significant.
There were 154 patients with monomicrobial Aeromonas bacteremia. The mean age was 58 yr (range, 24 to 92 yr) and the overall duration of hospitalization was 15 days (range, 1-82). There were 112 (73%) male patients and 43 (28%) patients with healthcare-associated bacteremias. There were 63 (41%) patients receiving acid-suppressant therapy. Liver cirrhosis was most common underlying disease (58%), followed by diabetes mellitus (28%) and solid cancer (26%). There were 126 (82%) patients with fever, 107 (69%) patients with thrombocytopenia, and only 40 (26%) patients with leukocytosis. Of the 87 (56%) patients with A. hydrophila bacteremia, one presented with acute cholangitis, one with spontaneous bacterial peritonitis, two with traumatic wound infections, two with urosepsis, four with necrotizing fasciitis, and the others with primary bacteremia. Of the 45 (29%) patients with A. veronii biovar sobria bacteremia, one presented with urosepsis, one with spontaneous bacterial peritonitis, one with necrotizing fasciitis, one with meningitis and the others with primary bacteremia. Of the 22 (14%) patients with A. caviae bacteremia, one patient presented with acute cholecystitis, one with traumatic wound infection, one with lung abscess and the others with primary bacteremia. There were 55 patients who died during hospitalization. Three patients with A. hydrophila bacteremia and one patient with A. caviae bacteremia survived more than 30 days after onset of Aeromonas bacteremia. Their deaths were considered not to be associated with Aeromonas bacteremia. One died due to hepatoma rupture, another due to esophageal veins bleeding, and the others due to septic shock caused by other pathogens. The remaining 51 patients' deaths were attributed to Aeromonas bacteremia.
Table 1 summarizes clinical presentations of the monomicrobial bacteremias caused by A. hydrophila, A. veroni biovar sobria, and A. caviae. A. caviae was more associated with healthcare-associated bacteremia than A. hydrophlia and A. veronii biovar sobria. However, A. veronii biovar sobria and A. hydrophila were more associated with the cirrhotic patients than A. caviae. Thrombocytopenia and shock were more common in A. veronii biovar sobria bacteremia and A. hydrophila bacteremia than A. caviae bacteremia. The APACHE II scores and mortality of A. hydrophila and A. veronii biovar sobria bacteremia were higher than A. caviae bacteremia.
Of the 87 A. hydrophila isolates, 61 were identified mainly by API-20NE system, 17 by Vitek II system, and 9 by BD-Phoenix system. Of the 45 A. veronii biovar sobria isolates, 34 were identified mainly by API-20NE system, 5 by Vitek II system, and 6 by BD-Phoenix system. Of the 22 A. caviae isolates, 16 were identified mainly by API-20NE system, 3 by Vitek II system, and 3 by BD-Phoenix system. In vitro antimicrobial susceptibilities of different Aeromonas species were listed in Table 2. More A. veronii biovar sobria isolates were susceptible to cefazolin and flomoxef than the other Aeromonas species. Less A. caviae isolates were susceptible to sulfamethoxazole/trimethoprim than the other Aeromonas species.
Univariate analyses for risk factors of bacteremia-associated mortalities were listed in Table 3. In A. hydrophila bacteremia, thrombocytopenia, diarrhea, APACHE II score > 20 points, and adequate empirical antibiotics were risk factors for bacteremia-associated mortality. The four factors were included for multivariate logistic regression analysis. Only APACHE II score was the independent factor for survival (odds ratio: 22.501; P < 0.001). In A. veronii biovar sobria bacteremia, APACHE II score was significant risk factor for bacteremia-associated mortality. Only one 77-yr-old woman with community-acquired A. caviae bacteremia died. She had liver cirrhosis, diabetes mellitus, and chronic usage of acid-suppressant therapy. She presented with fever, dyspnea, septic shock, and thrombocytopenia with 31 points of APACHE II score at admission. She was treated with levofloxacin, which was considered as an adequate empirical antibiotics according in vitro antimicrobial susceptibility. However, she still died after 24-day hospitalization. Fig. 1 showed box plots of the distributions of APACHE II scores for monomicrobial Aeromonas bacteremia stratified by different Aeromonas species and survival.
Commercial phenotyping systems used routinely in clinical microbiology laboratories are not exactly correct for identification of aeromonads (14, 15). Lamy and his coworkers compared 6 commercial systems for identifying clinical Aeromonas isolates (16). The accuracy of API-20NE system was good for A. hydrophila and A. veronii but not for A. caviae. The accuracy of Vitek II system for A. hydrophila and A. caviae was good but not for A. veronii. The accuracy of BD-Phoenix system for A. caviae and A. veronii was good but not for A. hydrophila. Additional tests, like esculin hydrolysis, gas production from glucose, Voges-Proskauer reaction, ornithine decarboxylase, and arginine dihydrolase production are necessary for confirmation of Aeromonas species identified by the commercial systems. However, their accuracy of identification is still not compatible with the molecular method. In the present study, large sample size alleviated this bias and complementary effect of these three commercial systems can decrease the extreme deviation caused by single commercial system.
The case mortality among patients with Aeromonas bacteremia in the literature ranges from 24%-63% (2-5, 17-22). Clinical presentations among different Aeromonas species were rarely discussed due to limited cases. In a study including 104 episodes, Aeromonas species was divided into hydrophila and non-hydrophila and the resulting fatalities were 35.5% (22/62) and 23.8% (10/42) respectively (2). In another report including 59 episodes, the mortalities caused by A. hydrophila, A. veronii biovar sobria, and A. caviae are 33% (13/40), 56% (5/9), and 17% (1/6) respectively (22). However, this tendency could not be noted in another report, in which Aeromonas-associated polymicrobial bacteremia was not excluded (4). The present study only included monomicrobial Aeromonas bacteremia and proved that the mortality of A. caviae bacteremia was lower than A. hydrophila bacteremia or A. veronii biovar sobria bacteremia.
Different Aeromonas species showed different virulence factors in immunocompromised mouse models (23). Majority of A. hydrophila and A. veronii biovar sobria isolates were capable of persistent colonization but A. caviae isolates was not. In vitro study showed that A. caviae isolates, unlike other Aeromonas isolates were less toxic to HEp-2 cell. However, most of Aeromonas isolates used for these studies were from the natural environment and the virulence factors of Aeromonas species from infected hosts and natural environments were different (24). Only a study showed the virulence factors from the bacteremia-associated Aeromonas isolates (12). The genes for cytotoxic enterotoxin were more common in the A. veronii biovar sobria (13/13) and A. hydrophila (15/20) isolates than the A. caviae isolates (3/14). Cytotoxic enterotoxin could activate mitogen-activated protein kinases and induce classical caspase-associated apoptosis in murine macrophages (25). Poor macrophage function caused by cytotoxic enterotoxin may contribute to severe sepsis. Therefore, poor abilities of A. caviae to produce cytotoxic enterotoxin may be the reason for better prognosis of A. caviae bacteremia. However, this opinion should be proved in a further study.
The patients diagnosed with healthcare-associated Aeromonas bacteremia had been considered to have colonization of Aeromonas species in their gastrointestinal tracts before admission (2). In the previous study, liver cirrhosis was associated with community-acquired Aeromonas bacteremia and malignancy with healthcare-associated Aeromonas bacteremia (2-4). In the present study, we found that A. caviae was more associated with healthcare-associated infection and less associated with cirrhosis than the other species. This phenomenon may be also due to poor abilities of A. caviae to produce cytotoxic enterotoxin. Cirrhotic patients have impaired intestinal permeability due to intestinal congestion, edema, and local hypoxia due to portal hypertension, which creates a good environment for bacterial translocation (26-29). However, additional factors for destroying mucosal barrier are necessary to help bacterial translocation. Cytotoxic enterotoxin produced by Aeromonas species can induce apoptosis of human intestinal epithelial cells and may play an important role for bacterial translocation (25). Due to poor production of enterotoxin, A. caviae has lower chance to cause bacterial translocation in cirrhotic patients than the other Aeromonas species. Compared with the cirrhotic patients, the cancer patients had more chances to receive surgeries or cytotoxic agents during hospitalization, which caused extensive intestinal mucosal damage. That may be the reason why A. caviae was more associated with healthcare-associated bacteremia.
In our study, the susceptibility of these microorganisms to trimethoprim-sulfamethoxazole, and cefazolin showed inter-species variability. These findings agree with previous studies (10, 11). Besides, we observed that flomoxef was active to only about 50% isolates of A. caviae and A. hydrophila. Although flomoxef belongs to oxyimino-β-lactam and is considered as a kind of extended-spectrum cephalosporin, traditional extended-spectrum cephalosporins are more efficacious for treatment of Aeromonas bacteremia.
Although there were some limitations from different commercial identifying systems in our study, this study included large sample size and showed different clinical presentations of bacteremia among A. hydrophila, A. veronii biovar sobria and A. caviae. In conclusion, the severity of A. caviae bacteremia is lower than A. hydrophila bacteremia or A. veronii biovar sobria bacteremia.
Figures and Tables
Fig. 1
Box plots of Acute Physiology and Chronic Health Evaluation II (APACHE II) scores distributions for A. hydrophila, A. veronii biovar sobria, and A. caviae bacteremia (A) and box plots of APACHE II scores distributions for survivals and deaths in different Aeromonas groups (B).

Table 1
Different clinical presentations among A. hydrophila, A. caviae and A. veronii biovar sobria bacteremia

Table 2
In vitro antimicrobial susceptibilities of different Aeromonas species
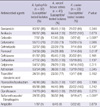
*More isolates of A. veronii biovar sobria were susceptible to cefazolin than A. hydrophila and A. caviae; †More isolates of A. veronii biovar sobria were susceptible to flomoxef than A. hydrophila and A. caviae; ‡Less isolates of A. caviae were susceptible to sulfamethoxazole/trimethoprim than A. hydrophila and A. veronii biovar sobria.
AUTHOR SUMMARY
Different Clinical Characteristics Among Aeromonas hydrophila, Aeromonas veronii biovar sobria and Aeromonas caviae Monomicrobial Bacteremia
Han-Chuan Chuang, Yu-Huai Ho, Chorng-Jang Lay, Lih-Shinn Wang, Yeong-Shu Tsai and Chen-Chi Tsai
This study aimed to evaluate the clinical presentations of 154 patients having A. hydrophila, A. veronii biovar sobria or A. caviae monomicrobial bacteremia by retrospective methods in Taiwan. The clinical presentation of A. caviae bacteremia was much different from A. hydrophila and A. veronii biovar sobria bacteremia. The patients with A. caviae bacteremia had less severity and lower mortality than those with A. veronii biovar sobria or A. hydrophila bacteremia.
References
1. Hazen TC, Fliermans CB, Hirsch RP, Esch GW. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl Environ Microbiol. 1978. 36:731–738.
2. Ko WC, Lee HC, Chuang YC, Liu CC, Wu JJ. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J Infect. 2000. 40:267–273.
3. Lay CJ, Zhuang HJ, Ho YH, Tsai YS, Wang LS, Tsai CC. Different clinical characteristics between polymicrobial and monomicrobial Aeromonas bacteremia: study of 216 cases. Intern Med. 2010. 49:2415–2421.
4. Kang JM, Kim BN, Choi SH, Kim NJ, Woo JH, Ryu J, Kim YS. Clinical features and prognostic factors of Aeromonas bacteremia. Infect Chemother. 2005. 37:161–166.
5. Sherlock CH, Burdge DR, Smith JA. Does Aeromonas hydrophila preferentially colonize the bowels of patients with hematologic malignancies? Diagn Microbiol Infect Dis. 1987. 7:63–68.
6. Krzymińska S, Kaznowski A, Lindner K, Mnichowska M. Enteropathogenic activity and invasion of HEp-2 cells by Aeromonas caviae clinical isolates. Acta Microbiol Pol. 2003. 52:277–283.
7. Laohachai KN, Bahadi R, Hardo MB, Hardo PG, Kourie JI. The role of bacterial and non-bacterial toxins in the induction of changes in membrane transport: implications for diarrhea. Toxicon. 2003. 42:687–707.
8. Janda JM. Biochemical and exoenzymatic properties of Aeromonas species. Diagn Microbiol Infect Dis. 1985. 3:223–232.
9. Dryden M, Munro R. Aeromonas septicemia: relationship of species and clinical features. Pathology. 1989. 21:111–114.
10. Motyl MR, McKinley G, Janda JM. In vitro susceptibilities of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae to 22 antimicrobial agents. Antimicrob Agents Chemother. 1985. 28:151–153.
11. Burgos A, Quindós G, Martínez R, Rojo P, Cisterna R. In vitro susceptibility of Aeromonas caviae, Aeromonas hydrophila and Aeromonas sobria to fifteen antibacterial agents. Eur J Clin Microbiol Infect Dis. 1990. 9:413–417.
12. Wu CJ, Wu JJ, Yan JJ, Lee HC, Lee NY, Chang CM, Shih HI, Wu HM, Wang LR, Ko WC. Clinical significance and distribution of putative virulence markers of 116 consecutive clinical Aeromonas isolates in southern Taiwan. J Infect. 2007. 54:151–158.
13. Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Document M45-A. 2006. Wayne, PA: Clinical and Laboratory Standards Institute.
14. Abbott SL, Cheung WK, Janda JM. The genus Aeromonas: biochemical characteristics, atypical reactions, and phenotypic identification schemes. J Clin Microbiol. 2003. 41:2348–2357.
15. Ormen O, Granum PE, Lassen J, Figueras MJ. Lack of agreement between biochemical and genetic identification of Aeromonas spp. APMIS. 2005. 113:203–207.
16. Lamy B, Laurent F, Verdier I, Decousser JW, Lecaillon E, Marchandin H, Roger F, Tigaud S, de Montclos H, Kodjo A. colBVH Study Group. Accuracy of 6 commercial systems for identifying clinical Aeromonas isolates. Diagn Microbiol Infect Dis. 2010. 67:9–14.
17. Duthie R, Ling TW, Cheng AF, French GL. Aeromonas septicaemia in Hong Kong species distribution and associated disease. J Infect. 1995. 30:241–244.
18. Janda JM, Guthertz LS, Kokka RP, Shimada T. Aeromonas species in septicemia: laboratory characteristics and clinical observations. Clin Infect Dis. 1994. 19:77–83.
19. Funada H, Matsuda T. Aeromonas bacteremia in patients with hematologic diseases. Intern Med. 1997. 36:171–174.
20. Harris RL, Fainstein V, Elting L, Hopfer RL, Bodey GP. Bacteremia caused by Aeromonas species in hospitalized cancer patients. Rev Infect Dis. 1985. 7:314–320.
21. Lee LN, Luh KT, Hsieh WC. Bacteremia due to Aeromonas hydrophila: a report of 40 episodes. Taiwan Yi Xue Hui Za Zhi. 1986. 85:123–132.
22. Ko WC, Chuang YC. Aeromonas bacteremia: review of 59 episodes. Clin Infect Dis. 1995. 20:1298–1304.
23. Lye DJ, Rodgers MR, Stelma G, Vesper SJ, Hayes SL. Characterization of Aeromonas virulence using an immunocompromised mouse model. Curr Microbiol. 2007. 54:195–198.
24. Yucel N, Erdogan S. Virulence properties and characterization of aeromonads isolated from foods of animal origin and environmental sources. J Food Prot. 2010. 73:855–860.
25. Galindo CL, Fadl AA, Sha J, Gutierrez C Jr, Popov VL, Boldogh I, Aggarwal BB, Chopra AK. Aeromonas hydrophila cytotoxic enterotoxin activates mitogen-activated protein kinases and induces apoptosis in murine macrophages and human intestinal epithelial cells. J Biol Chem. 2004. 279:37597–37612.
26. Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995. 108:1835–1841.
27. Quigley EM. Gastrointestinal dysfunction in liver disease and portal hypertension. Gut-liver interactions revisited. Dig Dis Sci. 1996. 41:557–561.
28. Such J, Guardiola JV, de Juan J, Casellas JA, Pascual S, Aparicio JR, Solá-Vera J, Pérez-Mateo M. Ultrastructural characteristics of distal duodenum mucosa in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2002. 14:371–376.
29. Chiva M, Guarner C, Peralta C, Llovet T, Gómez G, Soriano G, Balanzó J. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003. 15:145–150.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download