Abstract
This study was done to observe the alteration of the estimated glomerular filtration rate (eGFR) in multiple myeloma patients according to type of tandem hematopoietic stem cell transplantation (HSCT). Forty-one patients were enrolled in this study. Twenty patients underwent autologous HSCT (auto-HSCT) and 21 patients underwent allogeneic HSCT (allo-HSCT). The changes in eGFR after the two tandem HSCT modalities were different between the two groups, according to the donor of stem cells (P = 0.016). In the auto-HSCT group, the eGFR, recorded 12 months after secondary HSCT, was significantly decreased compared with the eGFR recorded before stem cell mobilization (P = 0.005). Although there was no significant difference, the trend showed that the eGFR after allo-HSCT decreased from the previous HSCT until a month after secondary HSCT. In addition, after 6 months of secondary HSCT, the eGFR recovered to the level recorded prior to the HSCT (P = 0.062). This difference may be due to total body irradiation, a calcineurin inhibitor, or maintemance therapy. Changes in renal function would be monitored closely for these patients. The recovery of the eGFR would be a main focus for the patients treated with the total body irradiation or the calcineurin inhibitor, a progressive decline of the eGFR would be also crucial for the patients treated with maintenance therapy.
Multiple myeloma is a clonal B-cell disease of slowly proliferating plasma cells, which is accompanied by the production of monoclonal proteins and lytic bone lesions (1). Up to half of newly diagnosed patients have shown a decrease in creatinine clearance and approximately 9% require dialysis because of severe renal impairments (1). Renal failure is contributed by several factors including monoclonal light chains, hypercalcemia, infection, and hyperuricemia (1). Cast nephropathy is a typical renal complication found in myeloma patients.
Hematopoietic stem-cell transplantation (HSCT) is an effective therapy for diseases that require strong chemotherapy such as hematopoietic malignancies, some solid tumors, and other immune disorders. However, renal problems are common after HSCT, with a cumulative incidence of an acute kidney injury (AKI) and a chronic kidney disease (CKD) holding 30%-50% and 17.5%-66% of cases, respectively (2). Renal problems after HSCT have been associated with the administration of nephrotoxic drugs, ischemia, radiation, and graft-versus-host disease (GVHD).
In multiple myeloma, tandem HSCT improves overall survival compared with single HSCT (3). Tandem HSCT is currently considered a standard option for multiple myeloma patients. Depending on the donor of the stem cells, tandem HSCT consists of autologous tandem HSCT (auto-HSCT) or autologous/allogeneic tandem HSCT (allo-HSCT). Some studies have shown that HSCT performed in patients with multiple myeloma reversed renal failure (4). However, there are few reports on the alteration of an estimated glomerular filtration rate (eGFR) in multiple myeloma patients after the two types of tandem HSCT. The aim of this study was to evaluate the changes in renal function after different tandem HSCT approaches in patients with multiple myeloma.
We reviewed the medical records at Seoul and Yeouido St. Mary's Hospital in Korea and identified 138 myeloma patients who had undergone high-dose chemotherapy and HSCT between August 1999 and March 2009. For the purpose of ruling out the effect of factors associated with changes in renal function, we excluded subjects who had co-morbid conditions (n = 41) such as diabetes or hypertension, who had undergone single HSCT (n = 41), who had less than 12-month-follow-up or relapse within 12 months (n = 14), or who had undergone renal replacement therapy before HSCT (n = 1). Therefore, 41 patients were enrolled in this study. AKI following HSCT was defined as ≥ 2-fold increase in serum creatinine within 100 days (5). All patients in this study did not develop AKI during transplantation.
Peripheral stem cells were mobilized and collected after the administration of granulocyte colony-stimulating factor (G-CSF) and cyclophosphamide (2 g/m2). For the first autologous HSCT, patients received melphalan (90-100 mg/m2/day for 2 days). The second HSCT was scheduled within 4-10 months. According to the donor of the stem cells, patients were divided into auto-HSCT and allo-HSCT groups. The followings clinical and laboratory data were collected within a month of the date of stem cell mobilization (SCM): gender, any symptom or sign at diagnosis, immunoglobulin type, time from diagnosis to HSCT, chemotherapy regimens prior to HSCT, body mass index, serum creatinine, and an eGFR. The staging at diagnosis was carried out according to the Durie/Salon staging system (6). The data collected during follow-up included age at HSCT, conditioning regimens for HSCT, donor of hematopoietic stem cells, nephrotoxic drugs during HSCT, and presence of cytomeglovirus (CMV) infection and GVHD. All patients who underwent allo-HSCT received a calcineurin inhibitor after secondary HSCT. Clinicians adjusted the calcineurin inhibitor to maintain a trough level (166-333 nM/L in cyclosporine and 12-25 nM/L in tacrolimus). They were tapered according to the clinical settings. An eGFR was measured before the first HSCT, a month after the first HSCT, and 1, 3, 6, 9, 12, 18, and 24 months after the secondary HSCT. The eGFR was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation (7). CKD stages were defined according to the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guideline (8).
The data were analyzed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). Data were presented as means (range) or as counts and percentages. For continuous variables, means were compared using an independent t test. Pearson's chi-squared test or Fisher's exact test were used for categorized variables. We used repeated measures ANOVA for the trend in eGFR after HSCT. P < 0.05 was considered significant.
Twenty patients underwent auto-HSCT and 21 patients underwent allo-HSCT. Among them, 12 patients had been described in previous papers (9, 10). As shown in Table 1, there were no significant differences in gender, mean age at HSCT, time from diagnosis to transplant, and symptoms and stage at diagnosis. Intact immunoglobulin was the most common isotype of immunoglobulin in both groups. Chemotherapy regimens prior to auto-HSCT were vincristine, doxorubicin and dexamethasone (VAD) in eight patients (40%); vincristine, epirubicin and dexamethasone (VED) in five patients (25%); bortezomib with dexamethasone (VD) in one patient (5%); thalidomide with dexamethasone in one patient (5%); bortezomib, doxorubicin and dexamethasone (PAD) in one patient (5%); and multiple regimens in four patients (20%). Chemotherapy regimens prior to allo-HSCT were VAD in seven patients (33.3%); VED in four patients (19%); doxorubicin, bortezomib, dexamethasone, and thalidomide (PTAD) in three patients (14.3%); VD in one patient (4.8%), PAD in one patient (4.8%); and multiple regimens in five patients (23.8%).
The levels of plasma creatinine and an eGFR before SCM were not significantly different between the two groups. The most common conditioning regimen was melphalan and total body irradiation (TBI) in auto-HSCT and fludarabine combined with melphalan in allo-HSCT. Among the patients who underwent auto-HSCT, 10 patients (50%) who received TBI were conditioned with 10-12 gray. None of the patients who underwent allo-HSCT received TBI. The fraction of patients treated with nephrotoxic agents was not significantly different between the two groups. Among the 21 patients who underwent allo-HSCT, seven patients had acute GVHD (33.3%), and 18 patients had chronic GVHD (85.7%). One patient (5%) in the auto-HSCT group and eight patients (38.1%) in the allo-HSCT group were treated for CMV infection within 1 yr after HSCT.
Among the patients who underwent auto-HSCT, 16 patients (80%) received maintenance therapy after HSCT. The maintenance therapy was started 3.6 ± 2.8 months after a secondary HSCT. The regimens of maintenance therapy were interferon-α in three patients (15%); thalidomide in three patients (15%); interferon-α with steroid in three patients (15%); thalidomide with steroid and zoledronic acid in three patients (15%); thalidomide with steroid in one patient (5%); zoledronic acid with steroid in one patient (5%); interferon-α with thalidomide in one paitent (5%); and cyclophosphamide with thalidomide and zoledronic acid in one patient (5%). None of the patients who underwent allo-HSCT received maintenance therapy.
The changes in eGFR after the two tandem HSCT modalities were different between the two groups, according to the donor of the stem cells (P = 0.016) (Fig. 1). In the auto-HSCT group, the eGFR recorded 12 months after secondary HSCT was significantly decreased compared with the eGFR recorded before SCM (P = 0.005). Although there was no significant difference, the trend showed that the eGFR after allo-HSCT decreased until a month after the secondary HSCT. After 6 months of secondary HSCT, the eGFR recovered to the level recorded prior to the HSCT (P = 0.062).
Comparing the evolution of renal function in those who received TBI and those who did not among the auto-HSCT, the trend of the eGFR showed that eGFR in the two groups decreased consistently. However, the decrease of the eGFR was higher in those who received TBI (before SCM, 106.6 [89.2-137.3] for those who received TBI, 94.1 [60.0-123.4] for those who did not; 24 months after the secondary HSCT, 82.2 [66.3-94.5] for those who received TBI, 76.6 [45.0-93.8] for those who did not; P = 0.043). In patients who received TBI, the eGFR, recorded 12 months after secondary HSCT, was significantly decreased compared with the eGFR recorded a month after the secondary HSCT (104.8 [85.3-117.2] for a month after the secondary HSCT, 87.1 [67.0-102.8] for 12 months after the secondary HSCT; P = 0.006). Comparing the evolution of renal function in those who received the maintenance therapy and those who did not among the auto-HSCT, the changes in eGFR were not significant between the two groups (P = 0.443).
The stages 2 or 3 CKD before HSCT were 5 patients (25%) in auto-HSCT and 9 (42.9%) in allo-HSCT. The stages 2 or 3 CKD recorded 6 months after the secondary HSCT were 11 patients (55.0%) in auto-HSCT and 16 (76.2%) in allo-HSCT. The stages 2 or 3 CKD recorded 24 months after the secondary HSCT were 16 patients (80.0%) in auto-HSCT and 13 (61.9%) in allo-HSCT (Fig. 2). In allo-HSCT group, the eGFR of patients with stage IIIb at the time of diagnosis was 99.5 (60.6-123.4) before SCM and 81.9 (56.65-97.43) for 24 months after the secondary HSCT.
This study shows that the changes in eGFR varied according to the donor of HSCT. For the purpose of ruling out the effect of factors associated with changes in renal function, we only included subjects who did not develop AKI during transplantation, did not have co-morbid conditions such as diabetes or hypertension, or did not relapse within 12 months. The eGFR recorded after auto-HSCT decreased significantly between before and 12 months after the secondary HSCT. The eGFR recorded after allo-HSCT decreased significantly between before and a month after the secondary HSCT. Subsequently, the eGFR of this group was maintained during the first 6-month period and recovered after 6 months of the secondary HSCT, to the level observed prior to the HSCT.
Renal impairment is a common feature of multiple myeloma (11, 12). Because of the increase in treatment-related toxicity and mortality, patients with renal insufficiency are frequently excluded from HSCT. However, some reports show that HSCT may reverse renal impairment in patients with multiple myeloma and the prognosis of patients with HSCT is not different from that of patients without renal involvement (4, 13). Nevertheless, previous studies have not addressed the changes in an eGFR according to the type of tandem HSCT. This study reported the evaluation of the continuous changes of the eGFR after different tandem HSCT approaches.
In this study, stage IIIa was the most common stage at the time of diagnosis. Intact immunoglobulin, especially IgG, accounted for 75.6% of cases. These results are similar to those reported previously (14, 15). Regarding drug regimens, VAD was the regimen used most commonly to induce remission of multiple myeloma. Bortezomib with or without dexamethasone was the most common first regimen for non-responder. Patients with very good partial remission or complete remission underwent HSCT.
The classification of the patients into two groups based on the donor of the stem cells revealed that the pattern of changes in an eGFR was quite different. The eGFR after auto-HSCT decreased slowly until 12 months after the secondary HSCT. It is assumed that the decline of eGFR may be due to the administration of nephrotoxic drugs. Patient survival or relapse-free survival rate in recipients of auto-HSCT was inferior to that observed in recipients of allo-HSCT (16). Many studies have shown that a maintenance treatment after auto-HSCT improves patient survival or relapse-free survival (17). Therefore, most patients who underwent auto-HSCT received maintenance therapy in this study. Interferon-α, thalidomide, and zoledronic acid are the main drugs of choice for the maintenance treatment (18, 19). Interestingly, in this study, patients in the auto-HSCT group exhibited a significant decrease in eGFR 12 months after the secondary HSCT compared with patients in the allo-HSCT group. It is now well known that the kidney is the main site for interferon degradation (20). In addition, many cases of renal dysfunction in patients treated with zoledronic acid have been reported, although, to date, thalidomide pharmacokinetics has rarely been studied in patients with renal dysfunction (21, 22). A recent report demonstrated that multiple myeloma patients receiving zoledronic acid combined with thalidomide had a higher risk of renal dysfunction (23). All of these drugs could induce renal dysfunction. Therefore, a maintenance treatment using these drug combinations may play a major role in the renal dysfunction observed in patients treated with auto-HSCT. In this study, subgroup analysis by maintenance therapy did not show a difference in evolution of renal function, because most patients who underwent auto-HSCT received maintenance therapy. To overcome this, studies are required to observe more patients.
The association between renal dysfunction and TBI has been recognized for over a decade and was confirmed in some studies (24, 25). This typically occurs 6 to 12 months after the start of TBI. Although the primary site of radiation injury has not been identified, TBI is toxic to arteriole, glomerulaus and tubular epithelium (24, 25). In addition, the cortical tubular damage follows from the vascular alterations caused by irradiation, leading to glomerulosclerosis (25). In our study, the half of patients who underwent auto-HSCT received TBI. This study shows that there was a significant decrease in the eGFR between the first and the twelfth month after the secondary HSCT in the patients who received TBI.
In contrast with the results for auto-HSCT, the eGFR after allo-HSCT decreased rapidly from the time of a secondary HSCT until a month later, and it was then sustained up to 6 months after the secondary HSCT. Twelve months after allo-HSCT, the eGFR recovered and reached the level observed prior to HSCT. We think that this pattern of the eGFR variation could be attributed to calcineurin inhibitors and CMV infection. The calcineurin inhibitor and CMV infection are associated with a reduction in renal function (26). This is attributed to an indirect effect of CMV, which causes endothelial dysfunction and subsequent glomerular impairment. In addition, calcineurin inhibitors used as prophylaxis for, or as treatment of, GVHD are associated with renal dysfunction (26). Considering the higher dose of calcineurin inhibitor used in the early period after HSCT, this is a possible cause of renal dysfunction.
Fludarabine is a purine analogue and a component of reduced intensity conditioning regimens (27). We think that the initial decline of the eGFR might not be due to this drug, because renal toxicity of fludarabine occurs in less than 5% of patients. There are few studies on the decline of renal function caused by GVHD. To date, GVHD has usually been related to glomerular diseases, especially membranous nephropathy and minimal change disease; therefore, it seems unlikely that GVHD is associated with renal dysfunction (28).
Some studies have shown HSCT performed in patients with multiple myeloma reversed renal failure (4, 29). A study showed that the development of dialysis-dependent renal failure in patients with myeloma can be reversed by HSCT after high levels of chemotherapy (29). Lee et al. demonstrated a dialysis duration ≤ 6 months prior to HSCT and a pretransplant creatinine clearance of > 10 mL/min were significant factors for renal function recovery (29). One patient excluded from our study underwent hemodialysis for 8 months prior to HSCT and renal failure was not reversed by HSCT.
Our study had an inherent limitation of a retrospective analysis and a small number of patients. Additionally, there was no significant difference in the two groups, but the trend showed a higher use of antibiotics or antifungal agents in auto-HSCT. These might be associated with the decline of renal function. Considering the rarity of the patients with tandem HSCT and many confounding factors associated with changes in renal function, prospective multi-center study with more patients to control for variables is required.
In summary, changes in eGFR varied according to the donor of HSCT. The eGFR recorded after allo-HSCT decreased rapidly from the time of HSCT until a month after the HSCT; subsequently, the eGFR recovered. The eGFR after auto-HSCT decreased slowly until the twelfth month after a secondary HSCT. This difference may be due to TBI, a calcineurin inhibitor, or maintemance therapy. Therefore, changes in renal function would be monitored closely for these patients. Just as the recovery of the eGFR will be a main focus on the patients treated with TBI or a calcineurin inhibitor, a progressive decline of the eGFR will be also crucial for the patients treated with maintenance therapy.
Figures and Tables
Fig. 1
Changes in eGFR after the two types of tandem HSCT. SCM, stem-cell mobilization; HSCT, hematopoietic stem-cell transplantation; 1°, 1 month after first hematopoietic stem-cell transplantation; eGFR, estimated glomerular filtration rate; auto-HSCT, autologous tandem hematopoietic stem-cell transplantation; allo-HSCT, autologous/allogeneic tandem hematopoietic stem-cell transplantation.

Fig. 2
Incidence of chronic kidney disease stage 2 or 3 after the two types of tandem HSCT: before HSCT, 25% for auto-HSCT, 42.9% for allo-HSCT; 6 months after secondary HSCT, 55.0% for auto-HSCT, 76.2% for allo-HSCT; 24 months after secondary, 80.0% for auto-HSCT, 61.9% for allo-HSCT. preHSCT, before hematopoietic stem-cell transplantation, auto-HSCT, autologous tandem hematopoietic stem-cell transplantation; allo-HSCT, autologous/allogeneic tandem hematopoietic stem-cell transplantation.

Table 1
Characteristics of multiple myeloma patients that underwent hematopoietic stem cell transplantation
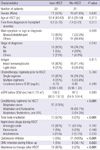
Data are expressed as numbers (percentages), means (range). auto-HSCT, autologous tandem hematopoietic stem-cell transplantation; allo-HSCT, autologous/allogeneic tandem hematopoietic stem-cell transplantation; HSCT, hematopoietic stem-cell transplantation; SCM, stem-cell mobilization; eGFR, estimated glomerular filtration rate; ATG, antithymocyte globulin; CMV, cytomegalovirus.
ACKNOWLEDGMENTS
This research was supported by Seoul St. Mary's Clinical Medicine Research, 2009, through the Catholic University of Korea.
AUTHOR SUMMARY
Changes in Renal Function after Different Tandem Hematopoietic Stem-cell Transplantation Approaches in Patients with Multiple Myeloma
Seok Hui Kang, Hyeon Seok Hwang, Hoon Suk Park, In O Sun, Sun Ryoung Choi, Byung Ha Chung, Bum Soon Choi, Chul Woo Yang, Yong Soo Kim, Chang Ki Min and Cheol Whee Park
There are few reports on the alteration of kidney function (estimated glomerular filtration rate [eGFR]) in multiple myeloma patients according to type of tandem hematopoietic stem cell transplantation (HSCT). In the auto-HSCT group (n = 20), the eGFR was significantly decrease. In contrast, the eGFR after allo-HSCT (n = 21) decreased initially, and then showed recovering tendency after 6 months of HSCT. This difference may be due to total body irradiation (TBI), calcineurin inhibitor, or maintemance therapy. It is requested to closely monitor the renal functions in those patients.
References
1. Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma: a demographic study of 1353 patients. Eur J Haematol. 1994. 53:207–212.
2. Chan GS, Lam MF, Au WY, Chim S, Tse KC, Lo SH, Fung SH, Lai KN, Chan KW. Clinicopathologic analysis of renal biopsies after haematopoietic stem cell transplantation. Nephrology. 2008. 13:322–330.
3. Attal M, Harousseau JL, Facon T, Guihot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003. 349:2495–2502.
4. Parikh GC, Amjad AI, Saliba RM, Kazmi SM, Khan ZU, Lahoti A, Hosing C, Mendoza F, Qureshi SR, Weber DM, Wang M, Popat U, Alousi AM, Champlin RE, Giralt SA, Qazilbash MH. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009. 15:812–816.
5. Parikh CR, McSweeney P, Schrier RW. Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int. 2005. 67:1999–2005.
6. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975. 36:842–854.
7. Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000. 11:155A.
8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002. 39:2 Suppl 1. S1–S266.
9. Eom KS, Min CK, Lee S, Kim YJ, Kim SY, Cho SG, Lee JW, Min WS, Kim CC. Pre-transplant disease status is important for an improved outcome of the second stem cell transplantation in the myeloma patients receiving the first autologous stem cell transplantation. Korean J Hematol. 2006. 41:36–40.
10. Jung SK, Kim MS, Lim JH, Kim YG, Han KJ, Min CK, Min WS. Serum free light chains for diagnosis and follow-up of multiple myeloma. Korean J Lab Med. 2008. 28:169–173.
11. Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma: pathogenesis and prognostic implications. Arch Intern Med. 1990. 150:1693–1695.
12. Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008. 22:1485–1493.
13. Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, Vesole DH, Jagannath S, Meyers R, Barlogie B. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996. 2:947–952.
14. Nair B, Waheed S, Szymonifka J, Shaughnessy JD Jr, Crowley J, Barlogie B. Immunoglobulin isotypes in multiple myeloma: laboratory correlates and prognostic implications in total therapy protocols. Br J Haematol. 2009. 145:134–137.
15. Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, Pérez-Simon JA, Kröger N, Moreau P, Gahrton G, Gasparetto C, Giralt S, Bensinger W. International Myeloma Working Group. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010. 28:4521–4530.
16. Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, Giaccone L, Sorasio R, Omedé P, Baldi I, Bringhen S, Massaia M, Aglietta M, Levis A, Gallamini A, Fanin R, Palumbo A, Storb R, Ciccone G, Boccadoro M. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007. 356:1110–1120.
17. Harousseau JL. Maintenance treatment in multiple myeloma. Ann Oncol. 2008. 19:Suppl 4. iv54–iv55.
18. Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA. National Cancer Research Institute Haematological Oncology Clinical Study Group. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010. 376:1989–1999.
19. Mihelic R, Kaufman JL, Lonial S. Maintenance therapy in multiple myeloma. Leukemia. 2007. 21:1150–1157.
20. Berenguer M. Treatment of chronic hepatitis C in hemodialysis patients. Hepatology. 2008. 48:1690–1699.
21. Diel IJ, Weider R, Köppler H, Antràs L, Smith M, Green J, Wintfeld N, Neary M, Duh MS. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review. Support Care Cancer. 2009. 17:719–725.
22. Izzedine H, Launay-Vacher V, Deray G. Thalidomide for the nephrologist. Nephrol Dial Transplant. 2005. 20:2011–2012.
23. Jones SG, Dolan G, Lengyel K, Myers B. Severe increase in creatinine with hypocalcemia in thalidomide-treated myeloma patients receiving zoledronic acid infusions. Br J Haematol. 2002. 119:576–577.
24. Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006. 17:1995–2005.
25. Borg M, Hughes T, Horvath N, Rice M, Thomas AC. Renal toxicity after total body irradiation. Int J Radiat Oncol Biol Phys. 2002. 54:1165–1173.
26. Kahan BD. Cyclosporine. N Engl J Med. 1989. 321:1725–1738.
27. Nunes R, Pasos-Coelho JL, Miranda N, Nave M, da Costa FL, Abecasis M. Reversible acute renal failure following single administration of fludarabine. Bone Marrow Transplant. 2004. 33:671.
28. Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol. 2006. 1:685–694.
29. Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R, Talamo G, Muwalla F, Anaissie E, Hollmig K, Tricot G. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 2004. 33:823–828.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download