Abstract
Necrotizing fasciitis is known to be a highly lethal infection of deep-seated subcutaneous tissue and superficial fascia. Reports of necrotizing fasciitis due to Streptococcus pneumoniae are exceedingly rare. We report a case of necrotizing fasciitis in a 62-yr-old man with liver cirrhosis and diabetes mellitus. He presented with painful swelling of left leg and right hand. On the day of admission, compartment syndrome was aggravated and the patient underwent surgical exploration. Intra-operative findings revealed necrotizing fasciitis and cultures of two blood samples and wound aspirates showed S. pneumoniae. The patient died despite debridement and proper antimicrobial treatment. To the best of our knowledge, this is the first case of fatal necrotizing fasciitis with meningitis reported in Korea. We also review and discuss the literature on pneumococcal necrotizing fasciitis.
As first defined by Wilson (1), necrotizing fasciitis is a highly lethal infection of deep-seated subcutaneous tissue and superficial fascia. Initial clinical characteristics usually include pain, swelling, and erythema, and as the disease progresses skin discoloration, bullae and necrosis are often observed. The mortality rate of necrotizing fasciitis ranges from 20% to 60% (2), and β-hemolytic streptococci or mixed infections of both aerobic and anaerobic flora are the usual causes.
Streptococcus pneumoniae is a common pathogen implicated in community-acquired pneumonia, sinusitis, otitis media and meningitis. However, necrotizing fasciitis caused by S. pneumoniae is uncommon.
In this paper, we describe a patient who presented with necrotizing fasciitis and meningitis from whom S. pneumoniae was isolated as the single pathogen. To the best of our knowledge, this is the first such case reported in Korea. We also review the literature on pneumococcal necrotizing fasciitis. We reviewed all English language articles published from 1970 to 2010 through MEDLINE using the following keywords: "necrotizing fasciitis," "soft tissue infection" and "Streptococcus pneumoniae."
A 62-yr-old man was hospitalized in a tertiary medical center in March 2010 with a five day history of pain and swelling in the left lower leg and progressing swelling over the right hand. He had developed alcoholic liver cirrhosis 27 yr prior, and was being treated for his condition with furosemide. One year prior to presentation, he was also diagnosed with type 2 diabetes mellitus. Seven days prior to admission, he ingested raw sea squirts. There was no significant travel history or contact with sick individuals.
On physical examination the patient was severely ill and had a temperature of 36.0℃. His blood pressure was 80/60 mmHg, and his pulse was 128 beats/min. Signs indicative of compartment syndrome involving the right hand and left leg were present, and skin discoloration with bullae was observed over the lateral malleolar area of his left leg (Fig. 1). Laboratory studies showed an elevated leukocyte count of 11,770/µL and thrombocytopenia (platelet count, 44,000/µL). There were signs of impaired coagulation, including a prolonged partial thromboplastin time of 50 sec (normal maximum, 36 sec) and an international normalized ratio of 1.6 (normal range, 0.90-1.20). The following values were also increased from normal levels: serum aspartate aminotransferase 82 U/L (normal range, 10-40 U/L); serum total bilirubin 2.63 mg/dL (normal range, 0.2-1.1 mg/dL); serum alkaline phosphatase 175 U/L (normal range, 50-128 U/L); creatinine 1.6 (normal range, 0.6-1.2 mg/dL); and creatine kinase 266 U/L (normal range, 60-220 U/L). Serologic tests were negative for HIV, hepatitis B and hepatitis C.
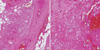
Compartment syndrome was aggravated and the patient underwent surgical exploration of the left lower leg and the dorsum of right hand 9 hr after admission. Intra-operative findings showed large areas of necrotic subcutaneous tissue and fascia but no pus formation (Fig. 2). The tissue planes were easily dissected with a gloved finger. Extensive debridement was conducted. Microscopic histopathology of the sample taken from the necrotic area during debridement is shown Fig. 3. Gross necrosis was observed throughout the soft tissue. There was inflammatory infiltration and thrombosis in the small vessels indicative of necrotizing fasciitis. Because of the patient's history of eating sea squirts, we could not exclude the possibility of vibrio infection, and antibiotic therapy with dose-adjusted cefotaxime (2 g every 12 hr) and doxycycline (100 mg every 12 hr) was administered preoperatively.
On hospital day 3, cultures of two blood samples and wound aspirates showed S. pneumoniae, so doxycycline was withdrawn. The E-test was used to determine the minimal inhibitory concentration (MIC) of the antibiotic. Antibiotic susceptibility testing of the isolate revealed MICs to penicillin of 0.75 µg/mL, cefotaxime 0.75 µg/mL, and ceftriaxone 0.5 µg/mL. Serological analysis revealed that the S. pneumoniae isolate belonged to serotype 9 V.
Despite aggressive daily surgical debridement, the necrosis extended to the other leg. On hospital day 6, a change in the patient's mental status occurred. No focal neurological deficits were noted. A lumbar puncture was performed, and the cerebrospinal fluid (CSF) had a protein concentration of 66 mg/dL, a glucose level of 52 mg/dL, a CSF-blood glucose ratio less than 0.25, 0 red blood cells/µL, and 42 white blood cells/µL (55% neutrophils and 18% lymphocytes). Therapy with vancomycin (1 g every 12 hr) was added and the final result of CSF culture was negative. Dialysis was begun on day 7. On hospital day 8, the patient died of septic shock and multi-organ failure.
Necrotizing fasciitis is not so rare, but necrotizing fasciitis due to S. pneumonia is exceedingly rare: we found only 19 reported cases, including the present report (3-17). Ages ranged from 21-83 yr, with an average of 50.1 yr (Table 1). Females were infected more often than males (male:female 7:12).
Predisposing factors of necrotizing fasciitis are an age of more than 50 yr, diabetes mellitus, drug abuse, hypertension, vascular diseases and obesity (18). The majority of these pneumococcal necrotizing fasciitis patients had immunocompromising conditions, such as systemic lupus erythematosus (10, 14, 16), diabetes mellitus (4, 5, 17), chronic renal failure (8, 15), chronic liver disease (4, 12), intravenous drug use (13) and cardiovascular disease (4). We also found reports of two patients who had undergone intramuscular injection with nonsteroidal anti-inflammatory drugs (6). The present findings agree with those of previous studies (19) that the common risk factors for invasive pneumococcal infections are alcoholism, splenectomy, connective tissue disease, steroid use, diabetes mellitus and intravenous drug use. Furthermore, these findings show that the immune system is an important defense against S. pneumoniae. Four of the patients described in previous studies had no underlying disease (3, 7, 9, 11), but all patients had experienced some form of trauma before admission. Therefore, small skin breaks due to blunt trauma are possible routes of inoculation, and any resulting hematoma might act as a nidus for the localization of S. pneumoniae infection (19).
The salient feature of pneumococcal necrotizing fasciitis is its predilection for lower extremity infection (14 patients). The five other cases involved the neck, flank and upper extremities (3, 4, 8, 14, 16). S. pneumoniae was isolated from the cultures of blood and site aspirations in 13 cases, while in 3 cases it was found only in the blood (10, 12, 14), and in 3 cases only in the local aspirates (3, 5, 7).
Even though there were the possibilities of report bias, which means more fatal cases had been reported, ten of the 19 patients died of pneumococcal necrotizing fasciitis (mortality rate, 52.6%). All three patients who did not receive aggressive surgical debridement died (4, 9, 10), which underscores the importance of early diagnosis and prompt surgical intervention.
The clinical characteristics of necrotizing fasciitis caused by group A β-hemolytic streptococci and S. pneumoniae are similar. They all occur frequently in elderly patients and in patients who are immunocompromised or have other underlying conditions. In the present case, the patient had liver cirrhosis and diabetes mellitus. Several antigenic molecular determinants such as Streptococcus pyrogenic exotoxins A, B and C encoded by the spe loci have been associated with necrotizing fasciitis due to group A β-hemolytic Streptococcus. Pneumococcal necrotizing fasciitis, on the other hand, causes an invasive infection through hyaluronidase and neuraminidase activity, which play roles in the migration of the organism through the fascial tissue (3). Moreover, there no DNA fragments corresponding to the spe gene were detected in three previously reported cases of pneum ococcal necrotizing fasciitis (4). Further studies are needed to define the pathogenesis and epidemiology of pneumococcal necrotizing fasciitis.
About 90 pneumococcal serotypes can potentially cause disease. Current pneumococcal vaccines contain a mixture of the capsular polysaccharides from the more common serotypes and are effective against invasive disease (20). To assess whether certain serogroups of S. pneumoniae are preferentially associated with specific necrotizing fasciitis, we analyzed the pneumococcal cases we found in the literature. A total of 7 cases included information about serotyping (3, 4, 8, 11). Serogroup 9 was the most often isolated (3 cases), while serogroup 5, 6A, 10A and 14 were also isolated from one patient each. All isolated serotypes except 6A are included in the 23-valent peumococcal vaccine.
Not much is currently known about the effect of pneumococcal vaccines on necrotizing fasciitis, and our analysis suggests a possible approach to preventing it. Pneumococcal vaccines may prevent not only common infections like pneumonia, but also necrotizing fasciitis in patients with predisposing factors as in the present case. We suggest that clinicians consider the use of pneumococcal vaccines for high risk patients.
In summary, we describe a case of necrotizing fasciitis caused by S. pneumoniae. To the best of our knowledge, this is the first such case report in Korea. Our case differs from the 18 other cases we reviewed in the literature in that our patient's necrotizing fasciitis was followed by meningitis. Host factors such as immunodeficiency or underlying diseases may affect the course and severity of pneumococcal necrotizing fasciitis. Vaccination and early implementation of optimal therapies may reduce the mortality rate of pneumococcal necrotizing fasciitis.
Figures and Tables
Fig. 1
Necrotizing fasciitis of the patient's left leg at admission. Note violet purpuras with unclear margins and bullae of lateral malleolar area.
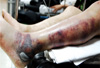
Fig. 2
Intra-operative findings: (A) right hand, (B) left lower leg. It was easy to separate the fascia from the subcutaneous tissue.

References
1. Wilson B. Necrotizing fasciitis. Am Surg. 1952. 18:416–431.
2. Dellinger EP. Severe necrotizing soft-tissue infections. Multiple disease entities requiring a common approach. JAMA. 1981. 246:1717–1721.
3. Ballon-Landa GR, Gherardi G, Beall B, Krosner S, Nizet V. Necrotizing fasciitis due to penicillin-resistant Streptococcus pneumoniae: case report and review of the literature. J Infect. 2001. 42:272–277.
4. Kwak EJ, McClure JA, McGeer A, Lee BC. Exploring the pathogenesis of necrotizing fasciitis due to Streptococcus pneumoniae. Scand J Infect Dis. 2002. 34:639–644.
5. Choudhri SH, Brownstone R, Hashem F, Magro CM, Crowson AN. A case of necrotizing fasciitis due to Streptococcus pneumoniae. Br J Dermatol. 1995. 133:128–131.
6. Frick S, Cerny A. Necrotizing fasciitis due to Streptococcus pneumoniae after intramuscular injection of nonsteroidal anti-inflammatory drugs: report of 2 cases and review. Clin Infect Dis. 2001. 33:740–744.
7. Ben M'Rad M, Brun-Buisson C. A case of necrotizing fasciitis due to Streptococcus pneumoniae following topical administration of nonsteroidal anti-inflammatory drugs. Clin Infect Dis. 2002. 35:775–776.
8. Imhof A, Maggiorini M, Zbinden R, Walter RB. Fatal necrotizing fasciitis due to Streptococcus pneumoniae after renal transplantation. Nephrol Dial Transplant. 2003. 18:195–197.
9. Clad A, Orlowska-Volk M, Karck U. Fatal puerperal sepsis with necrotising fasciitis due to Streptococcus pneumoniae. BJOG. 2003. 110:213–214.
10. Isik A, Koca SS. Necrotizing fasciitis resulting from Streptococcus pneumoniae in recently diagnosed systemic lupus erythematosus case: a case report. Clin Rheumatol. 2007. 26:999–1001.
11. Dawar M, Russell B, McClean K, Levett PN, Tyrrell GJ, Irvine J. A case of necrotizing fasciitis due to Streptococcus pneumoniae serotype 5 in Saskatchewan. Can J Infect Dis Med Microbiol. 2008. 19:69–71.
12. Yamashiro E, Asato Y, Taira K, Awazawa R, Yamamoto Y, Hagiwara K, Tamaki H, Uezato H. Necrotizing fasciitis caused by Streptococcus pneumoniae. J Dermatol. 2009. 36:298–305.
13. Lewis RJ, Richmond AS, McGrory JP. Diplococcus pneumoniae Cellulitis in drug addicts. JAMA. 1975. 232:54–55.
14. Hill MD, Karsh J. Invasive soft tissue infections with Streptococcus pneumoniae in patients with systemic lupus erythematosus: case report and review of the literature. Arthritis Rheum. 1997. 40:1716–1719.
15. Patel M, Ahrens JC, Moyer DV, DiNubile MJ. Pneumococcal soft-tissue infections: a problem deserving more recognition. Clin Infect Dis. 1994. 19:149–151.
16. DiNubile MJ, Albornoz MA, Stumacher RJ, Van Uitert BL, Paluzzi SA, Bush LM, Nelson SC, Myers AR. Pneumococcal soft-tissue infections: possible association with connective tissue diseases. J Infect Dis. 1991. 163:897–900.
17. Prakash PK, Biswas M, ElBouri K, Braithwaite PA, Hanna FW. Pneumococcal necrotizing fasciitis in a patient with Type 2 diabetes. Diabet Med. 2003. 20:899–903.
18. Francis KR, Lamaute HR, Davis JM, Pizzi WF. Implications of risk factors in necrotizing fasciitis. Am Surg. 1993. 59:304–308.
19. Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med. 1999. 107:12S–27S.
20. Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000. 30:122–140.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download