Abstract
Exhaled nitric oxide (eNO) has been proposed as a noninvasive marker of airway inflammation in asthma. In asthmatic patients, exhaled NO levels have been shown to relate with other markers of eosinophilic recruitment, which are detected in blood, sputum, bronchoalveolar lavage fluid and bronchial biopsy samples. The purpose of this study was to assess the possible relationship between eNO and allergic inflammation or sensitization in childhood asthma and allergic rhinitis. Subjects consisted of 118 asthmatic children, 79 patients with allergic rhinitis, and 74 controls. Their age ranged from 6 to 15 yr old. eNO level, peripheral blood eosinophil count, eosinophil cationic protein (ECP), serum total IgE level and specific IgE levels were measured. Methacholine challenge test and allergic skin prick test for common allergens were performed in all subjects. Atopic group (n = 206, 44.48 ± 30.45 ppb) had higher eNO values than non-atopic group (n = 65, 20.54 ± 16.57 ppb, P < 0.001). eNO level was significantly higher in patients with asthma (42.84 ± 31.92 ppb) and in those with allergic rhinitis (43.59 ± 29.84 ppb) than in healthy controls (27.01 ± 21.34 ppb, P < 0.001) but there was no difference between asthma and allergic rhinitis group. eNO also had significant positive correlations with Dermatophagoides pteronyssinus IgE level (r = 0.348, P < 0.001), Dermatophagoides farinae IgE level (r = 0.376, P < 0.001), and the number of positive allergens in skin prick test (r = 0.329, P = 0.001). eNO had significant positive correlations with peripheral blood eosinophil count (r = 0.356, P < 0.001), serum total IgE level (r = 0.221, P < 0.001), and ECP (r = 0.436, P < 0.001). This study reveals that eNO level is associated with allergic inflammation and the degree of allergic sensitization.
Allergic diseases, including asthma, continue to increase in prevalence in the world (1). A key feature in the pathogenesis of asthma is chronic airway inflammation, characterized by the presence of inflammatory cells and the release of inflammatory mediators in the airways (2). Airway inflammation can be detected by several methods such as bronchial biopsy, bronchoalveolar lavage or induced sputum (3). However, due to their invasive character or low practical applicability, these methods are not suitable for use in young children or in large study populations. The measurement of nitric oxide concentrations in exhaled air has recently been proposed as a non-invasive, simple and well tolerated test to assess airway inflammation in asthma, even in children (4).
Exhaled nitric oxide (eNO) is a free-radical gas of endogenous origin with multiple biological and pathophysiologic functions, detectable in exhaled air of human. eNO in asthmatic patients is mainly produced by inducible nitric oxide synthase expressed in bronchial epithelial cells and some inflammatory cells (5). Allergic sensitization may contribute to eNO in children with asthma via late-phase influx of eosinophils (6) and nitric oxide formation (7, 8) after aeroallergen exposure. The fact that eNO level increase in subjects with mild-to-moderate asthma (9), increase after a late asthmatic reaction to allergens (10), and decrease after subjects receive inhaled corticosteroids (9) suggests that eNO is closely associated with airway inflammation. In contrast, others suggested that atopy and airway inflammation represented by eNO were separate dimensions in the assessment of childhood asthma (11).
Therefore, the present study aimed to determine whether eNO could be related with allergic inflammation and sensitization in childhood asthma and allergic rhinitis.
The study included 271 children: 118 asthmatic children, 79 patients with allergic rhinitis, and 74 controls. Their age ranged from 6 to 15 yr old.
The diagnosis of asthma was made on the basis of American Thoracic Society (ATS) criteria (12). The children with asthma reported having typical wheezing or episodic shortness of breath, and showed either a positive methacholine challenge results (PC20 < 16 mg/mL) or an increase by at least 12% in forced expiratory volume in 1 sec (FEV1) after administration of 200 µg of salbutamol. Patents were being treated with inhaled β-agonist alone on an as-required basis, and had not used any controller anti-asthmatic therapy during the 3 months prior to the study. They had no respiratory infection in recent 4 weeks. The diagnosis of allergic rhinitis was made on the basis of history (watery rhinorrhea, blocked nose, sneezing, and nasal itching) and positive skin prick tests to common allergens or specific IgE concentration assessed as higher than 0.35 IU/mL for at least one allergen. None of the children had ever presented wheezing or received asthma medication. Control subjects had no asthma or allergic rhinitis symptoms and history. Their PC20 values were 16 or higher and dFEV1 was lower than 12%. Atopy was defined as a positive skin prick tests (wheal diameter > 3 mm) to 16 common allergens or specific IgE concentration assessed as higher than 0.35 IU/mL for at least one allergen or serum total IgE concedntraction > 150 IU/mL.
Exclusion criteria for patient recruitment were: 1) Patient who was under steroid treatment for the 4 weeks preceding the study and 2) Patient who had upper airway infection over the last 4 weeks.
eNO was measured using a fast response chemiluminescence analyser (CLD 88 exhalyzer, ECO MEDICS, Duernten, Switzerland) according to the ATS recommendations (13). Subjects exhaled at a constant flow rate (50 mL/s) from total lung capacity to residual volume without breath-holding. Measurement was taken before spirometry and methacholine challenge test. The mean value of 3 successive reproducible recordings was retained for statistical analysis.
Serum was collected and stored at -20℃ until assayed. Peripheral blood eosinophil counts were done by automated assay (NE-8000 system, Sysmex, Kobe, Japan). Total serum IgE and eosinophil cationic protein (ECP) were measured (CAP system, Pharmacia-Upjohn, Uppsala, Sweden) according to the manufacturer's instructions. Specific IgE test was performed on Dermatophagoides pteronyssinus (Der p), Dermatophagoides farina (Der f), Alternaria, Blatella germinica, cow's milk and egg white (CAP system, Pharmacia-Upjohn, Uppsala, Sweden). A specific IgE value greater than 0.35 kIU/L was considered positive. Skin prick test (Torii & Co; Tokyo, Japan) measured two types of house dust mites, cat and dog epithelium, and mold and pollen antigens. A saline solution was used as a negative control, and a 0.5% histamine HCl solution was used as a positive control. The wheal diameter was measured after 15 min, and a positive reaction was defined as a wheal diameter of 3 mm (14).
All statistical tests were performed using the statistical analysis program SAS (version 9.1). Comparison of eNO values was performed using logistic regression between different groups. eNO values were log transformed before analysis to normalize residuals, and analyses were adjusted for age.
Pearson's correlation coefficients were used to assess the relationships among the eNO, biomarkers, and skin prick test result. The analyses were adjusted for the age.
A P value of less than 0.05 was considered significant.
Study subjects ranged in age from 6 to 15 yr (mean, 8.4). One hundred seventy four of them were male, and 97 were female. Atopic group consisted of 206 children, and non-atopic group 65 children. Atopic patients accounted for 83.1% of asthma group, 100% of allergic rhinitis, and 39.2% of control group.
Atopic group (44.48 ± 30.45 ppb) had higher eNO values than non-atopic group (20.54 ± 16.57 ppb, P < 0.001) (Fig. 1). Within asthma group, atopic asthma group (48.33 ± 32.28 ppb) had higher eNO values than non-atopic asthma group (15.92 ± 5.99 ppb, P = 0.002).
eNO level was significantly higher in patients with asthma (42.84 ± 31.92 ppb, P < 0.001) and in those with allergic rhinitis (43.59 ± 29.84 ppb, P < 0.001) than in healthy controls (27.01 ± 21.34 ppb). However, there was no difference between asthma and allergic rhinitis group.
eNO had a significant positive correlation with serum total IgE level (r = 0.221, P < 0.001). eNO also had positive correlations with Der p IgE level (r = 0.348, P < 0.001) and Der f IgE level (r = 0.376, P < 0.001) (Fig. 2). In addition, there was a positive correlation between eNO and the number of positive skin prick test (r = 0.329, P = 0.001) (Fig. 3).
eNO had positive correlations with peripheral blood eosinophil count (r = 0.356, P < 0.001) and ECP (r = 0.436, P < 0.001) (Fig. 4).
The major finding of this study is that increased eNO levels have been observed in atopic subjects regardless of underlying diseases. eNO levels were higher in asthma group and allergic rhinitis group than control group. There was a significant positive correlation between eNO with peripheral blood eosinophil count, ECP, total IgE, Der p- and Der f-specific IgE, and the number of positive skin prick test.
eNO has been widely accepted as a marker of chronic airway inflammation (15). Positive correlations have been reported between eNO and eosinophils in sputum (16), bronchial biopsy specimens (17) but have not been observed in some other studies (18).
Relationships between eNO and allergic sensitization have been previously reported. eNO correlates with total IgE and specific IgE to house dust mites, and there is a moderate correlation with the number of positive skin prick tests (19, 20) and wheal size (21). Several studies have reported elevated eNO levels in atopic subjects, regardless of the presence of respiratory tract symptoms (19, 20).
In contrast, some authors failed to find a relationship between eNO and allergic sensitization (11, 22). Leung et al. (11) suggested atopy and airway inflammation to represent separate dimensions of childhood asthma, using factor analysis. However, in that study, 59% of patients received inhaled corticosteroids. eNO level can decrease after subjects receive inhaled corticosteroids (9, 23). Therefore, inhaled corticosteroids treatment could influence the results. Persson et al. (22) found no difference in a small group of subjects in peak eNO concentration between allergic and nonallergic asthmatics. However, the number of subjects was very small, and 23 patients of 34 asthmatics were receiving regular inhaled corticosteroid treatment as well. In our study, we showed a positive correlation between eNO and allergic sensitization in 271 subjects, and none of them received corticosteroid treatment.
We used CLD 88 device to measure eNO level which is different from that commonly used one approved by FDA. However, in our previous study (24), we compared the value of eNO measured by CLD88 and Niox mino® (FDA approved), the intraclass correlation coefficient was 0.786 (P < 0.001). Therefore our data may not be derived from device difference.
eNO tends to increase in healthy children up to the age of 17 yr; thereafter the values are similar to adults (25). The tendency may be caused by increased airway size (25). Our findings are in agreement with this tendency. Therefore, we adjusted our data for age in statistical analysis.
We showed that eNO levels were correlated with allergic inflammation in children. However, we think this finding is not limited in pediatric group. There are some similar reports about the relationship between eNO and allergic inflammation in adult group (26).
In this study, asthma with allergic rhinitis group did not show higher eNO levels than asthma only or allergic rhinitis only group, but eNO levels of allergic rhinitis group were as high as those of asthma group. So we should be careful when we use eNO as a diagnostic or monitoring tool for asthma. Exhaled eNO level elevation in allergic rhinitis subjects probably results from allergic sensitization itself and a subclinical eosinophilic inflammatory process throughout the respiratory tract (27), indicating that rhinitis and asthma represent the manifestation of one syndrome with a wide spectrum of severity (28).
The findings of this study suggests that the allergic nature of inflammation of the airways may be mainly responsible for higher NO production in the airways. In conclusion, our study shows that eNO level correlates with allergic inflammation and the degree of allergic sensitization.
Figures and Tables
Fig. 1
Comparison of eNO level between atopic asthma, allergic rhinitis (AR), atopic control, non-atopic asthma, and non-atopic control. Atopic group (44.48 ± 30.45 ppb) had higher eNO values than non-atopic group (20.54 ± 16.57 ppb) (P < 0.001).

Fig. 2
Correlation between serum total IgE and eNO levels (A), specific IgE of Der p and eNO level (B), and specific IgE of Der f and eNO level (C). eNO had positive correlations with serum total IgE level (r = 0.221, P < 0.001), Der p IgE level (r = 0.348, P < 0.001), and Der f IgE level (r = 0.376, P < 0.001).
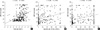
AUTHOR SUMMARY
Exhaled Nitric Oxide is Associated with Allergic Inflammation in Children
Bong Seok Choi, Kyung Won Kim, Yong Ju Lee, Jiyoung Baek, Hyun Bin Park, Yoon Hee Kim, Myung Hyun Sohn and Kyu-Earn Kim
We assessed the possible relationship between eNO and allergic inflammation in childhood asthma and allergic rhinitis. Atopic group had higher eNO values than non-atopic group. eNO level was significantly higher in patients with asthma and in those with allergic rhinitis than in healthy controls. eNO also had significant positive correlations with house dust mite IgE level, and the number of positive allergens in skin prick test. eNO had significant positive correlations with peripheral blood eosinophil count, serum total IgE level, and ECP. As a whole, eNO level is well associated with allergic inflammation and the degree of allergic sensitization.
References
1. Yunginger JW, Reed CE, O'Connell EJ, Melton LJ 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964-1983. Am Rev Respir Dis. 1992. 146:888–894.
2. Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med. 2005. 11:148–152.
3. Kim ES, Kim SH, Kim KW, Park JW, Kim YS, Sohn MH, Kim KE. Basement membrane thickening and clinical features of children with asthma. Allergy. 2007. 62:635–640.
4. Baraldi E, Azzolin NM, Zanconato S, Dario C, Zacchello F. Corticosteroids decrease exhaled nitric oxide in children with acute asthma. J Pediatr. 1997. 131:381–385.
5. Hamid Q, Springall DR, Riveros-Moreno V, Chanez P, Howarth P, Redington A, Bousquet J, Godard P, Holgate S, Polak JM. Induction of nitric oxide synthase in asthma. Lancet. 1993. 342:1510–1513.
6. Cieslewicz G, Tomkinson A, Adler A, Duez C, Schwarze J, Takeda K, Larson KA, Lee JJ, Irvin CG, Gelfand EW. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999. 104:301–308.
7. Roberts G, Hurley C, Bush A, Lack G. Longitudinal study of grass pollen exposure, symptoms, and exhaled nitric oxide in childhood seasonal allergic asthma. Thorax. 2004. 59:752–756.
8. de Kluijver J, Evertse CE, Schrumpf JA, van der Veen H, Zwinderman AH, Hiemstra PS, Rabe KF, Sterk PJ. Asymptomatic worsening of airway inflammation during low-dose allergen exposure in asthma: protection by inhaled steroids. Am J Respir Crit Care Med. 2002. 166:294–300.
9. Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994. 343:133–135.
10. Kharitonov SA, O'Connor BJ, Evans DJ, Barnes PJ. Allergen-induced late asthmatic reactions are associated with elevation of exhaled nitric oxide. Am J Respir Crit Care Med. 1995. 151:1894–1899.
11. Leung TF, Wong GW, Ko FW, Lam CW, Fok TF. Clinical and atopic parameters and airway inflammatory markers in childhood asthma: a factor analysis. Thorax. 2005. 60:822–826.
12. American Thoracic Society. Medical Section of the American Lung Association. Guidelines for the evaluation of impairment/disability in patients with asthma. Am Rev Respir Dis. 1993. 147:1056–1061.
13. American Thoracic Society. Medical Section of the American Lung Association. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. Am J Respir Crit Care Med. 1999. 160:2104–2117.
14. Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979. 63:328–335.
15. Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006. 61:817–827.
16. Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006. 174:1286–1291.
17. Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001. 164:1376–1381.
18. Lemière C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, Martin JG, Hamid Q. Airway inflammation assessed by invasive and noninvasive means in severe asthma: eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006. 118:1033–1039.
19. Saito J, Inoue K, Sugawara A, Yoshikawa M, Watanabe K, Ishida T, Ohtsuka Y, Munakata M. Exhaled nitric oxide as a marker of airway inflammation for an epidemiologic study in schoolchildren. J Allergy Clin Immunol. 2004. 114:512–516.
20. Franklin PJ, Turner SW, Le Souëf PN, Stick SM. Exhaled nitric oxide and asthma: complex interactions between atopy, airway responsiveness, and symptoms in a community population of children. Thorax. 2003. 58:1048–1052.
21. Frank TL, Adisesh A, Pickering AC, Morrison JF, Wright T, Francis H, Fletcher A, Frank PI, Hannaford P. Relationship between exhaled nitric oxide and childhood asthma. Am J Respir Crit Care Med. 1998. 158:1032–1036.
22. Persson MG, Zetterström O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994. 343:146–147.
23. Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995. 152:800–803.
24. Lee JH, Choi BS, Baek JY, Lee YJ, Kim KW, Sohn MH, Kim KE. Comparison of exhaled nitric oxide analysers in childhood asthma. Pediatr Allergy Respir Dis. 2011. 21:17–23.
25. Pavord ID, Shaw D. The use of exhaled nitric oxide in the management of asthma. J Asthma. 2008. 45:523–531.
26. Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007. 176:238–242.
27. Hung CH, Hua YM, Hsu WT, Lai YS, Yang KD, Jong YJ, Chu YT. Montelukast decreased exhaled nitric oxide in children with perennial allergic rhinitis. Pediatr Int. 2007. 49:322–327.
28. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003. 111:1171–1183.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download