Abstract
The purpose of this study was to investigate clinical and immunological responses to Demodex on the ocular surface. Thirteen eyes in 10 patients with Demodex blepharitis and chronic ocular surface disorders were included in this study and treated by lid scrubbing with tea tree oil for the eradication of Demodex. We evaluated ocular surface manifestations and Demodex counts, and analyzed IL-1β, IL-5, IL-7, IL-12, IL-13, IL-17, granulocyte colony-stimulating factor, and macrophage inflammatory protein-1β in tear samples before and after the treatment. All patients exhibited ocular surface manifestations including corneal nodular opacity, peripheral corneal vascularization, refractory corneal erosion and infiltration, or chronic conjunctival inflammatory signs before treatment. After treatment, Demodex was nearly eradicated, tear concentrations of IL-1β and IL-17 were significantly reduced and substantial clinical improvement was observed in all patients. In conclusion, we believe that Demodex plays an aggravating role in inflammatory ocular surface disorders.
Demodex (class Arachnida, superorder Acariformes) is an elongated ectoparasite found on the human body surface including the face, cheeks, forehead, nose, and eyelids (1). There are many species of Demodex, but only D. folliculorum and D. brevis are found on the human body (2). In the eye, Demodex can be found on the eyelashes, the lash follicles, and the meibomian glands, and is thought to be associated with blepharitis, allergic conjunctivitis, and pathological corneal features (2-5). Recently, a high prevalence of Demodex in eyelashes with cylindrical dandruff has been reported and a method of evaluating ocular demodecosis by sampling and counting Demodex has been introduced (6, 7). In addition, Gao et al. (8, 9) reported the ocular Demodex-killing effects of tea tree oil (TTO) in vitro and in vivo, and introduced a new clinical treatment, lid scrubbing with TTO, that has proven effective for eradicating ocular demodecosis. Nevertheless, the pathogenesis of Demodex on the ocular surface remains unclear up to recently. It has been merely presumed that inflammatory or specific immune reactions may be associated with ocular demodecosis (4, 9).
Therefore, we evaluated the changes of the clinical manifestations and the levels of tear cytokines following the eradication of Demodex to verify its pathogenicity, and to investigate the mechanisms of immunological response against Demodex on the ocular surface.
Thirteen eyes with ocular demodecosis and chronic ocular surface disorders of 10 patients were enrolled in this study. The 10 patients included six women and four men, with an average age of 48.3 ± 18.9 yr (range, 14 to 70 yr). Demographic and other clinical features are summarized in Table 1.
All patients reported ocular surface irritation and showed signs of ocular surface inflammation including conjunctival injections lasting over six months despite extensive patient-specific treatment including preservative-free artificial tears, corticosteroids, autologous serum, acyclovir (only for herpetic keratitis), antibiotics, or lid scrubbing with baby shampoo. The medical records of all patients, including history of present illness and systemic diseases, were reviewed. All of the 10 patients underwent complete ophthalmologic examinations, including external photographs, microscopic Demodex examination, and tear sampling. The patients were treated with a four-week regimen of lid scrubbing with TTO for ocular demodecosis and maintained prior topical treatments during that time. Four weeks after the initiation of treatment, we compared ocular surface manifestations, Demodex counts, and levels of inflammatory cytokines in tear samples measured before and after treatment.
Ocular demodecosis was confirmed by microscopic examination of epilated lashes following the method described by Gao et al. (8) with some modifications. Briefly, two lashes with cylindrical dandruff per lid were sampled and were placed separately on a glass slide. Under a slit-lamp light microscope at a magnification of × 16, one drop of 20 µL saline was applied by pipette to the edge of the glass slide for lashes without retained dandruff. For lashes with retained heavy dandruff, 20 µL of 100% alcohol was added. For the former, the number of Demodex was counted immediately and for the latter the counting time was delayed up to 20 min to allow the cylindrical dandruff to dissolve and to stimulate the migration of embedded Demodex. The Demodex count was recorded as the total number of mites found in a total of four lashes per eye.
Weekly lid scrubs with 50% TTO were performed and daily lid scrubs with 10% TTO shampoo were advised for a minimum of four weeks, according to the method reported by Gao et al. (9). In brief, at the clinic, after a drop of 0.5% proparacaine, a cotton tip wetted in 50% TTO was used to scrub the lid margin and lash roots for three sessions with a 10-min interval between each scrub. The patients were instructed to continue scrubbing daily at home, and advised to close their eyes and massage their lids with medium pressure for three to five minutes using a cotton tip wetted in 10% TTO shampoo. After treatment, the skin was rinsed with clean water and dried with a towel. We advised patients to perform home lid scrubs twice daily.
Unstimulated tear fluid was collected from the inferior meniscus of each eye with the least possible irritation using a pre-weighed polyester wick (Transorb rods; American Filtrona, Richmond, VA, USA) to obtain tear samples, as previously described (10). Wicks were then placed into the end of a micropipette tip located within a 0.5 mL tube (Eppendorf, Fremont, CA, USA). The tear samples were immediately transported in an insulated cooler to a -80℃ freezer where they remained frozen until they were used for immunoassays. Tears were extracted from the saturated wicks by adding a volume of buffer (50 mM Tris/HCl, 0.15 M NaCl, 10 mM CaCl2, 0.005% Brij35, 0.02% sodium azide [pH 7.5]) 10 times greater than the original volume of the tear sample to the pipette and then centrifuging at 12,000 rpm for five minutes. The rods and pipette tips were carefully removed and the tear fluid aspirated. Cytokines and chemokines in these samples were analyzed using a Luminex® 200™ Total System (Invitrogen™, Carlsbad, CA, USA). The cytokines and chemokines analyzed included: IL-1β, IL-5, IL-7, IL-12, IL-13, IL-17, granulocyte-colony stimulating factor (G-CSF), and macrophage inflammatory protein-1β (MIP-1β). The concentrations of these factors in tears were calculated from standard curves of known concentrations of recombinant human cytokines.
Demodex was found in all patients (Fig. 1). All patients reported ocular surface discomfort lasting over six months. They showed ocular surface manifestations including large corneal nodular opacity (four eyes; Fig. 2A-C), refractory corneal erosion and infiltration (12 eyes; Fig. 2D-F), peripheral corneal vascularization (6 eyes; Fig. 2C, G, H), chronic palpebral conjunctival papillary hypertrophy (7 eyes; Fig. 2I), and chronic bulbar conjunctival injection (13 eyes). Three eyes had atopic keratoconjunctivitis, four eyes had allergic conjunctivitis, and two eyes had severe dry eye syndrome (dysfunctional tear syndrome level 3) (11). Four eyes had had herpes simplex stromal keratitis in the past.
After four weeks of weekly lid scrubs with 50% tea tree oil and daily lid scrubs with tea tree shampoo, the disappearance of dandruff was noted in all patients' eyelashes. Demodex was completely eradicated in 10 of 13 eyes. The mean Demodex count per eye was reduced significantly, from 3.8 ± 2.2 to 0.2 ± 0.4 at four weeks after the initiation of treatment (Table 2; P = 0.001). All patients showed improvement of bulbar conjunctival injection (13 eyes), conjunctival papillary hypertrophy (7 eyes), and corneal erosions and infiltrations (12 eyes). Peripheral corneal vascularizations and nodular corneal opacities were markedly faded in three of six eyes and two of four eyes (Fig. 3).
The mean tear concentrations of all inflammatory cytokines before and after treatment are presented in Table 2. Tear concentrations of IL-1β (P = 0.001) and IL-17 (P = 0.001) were significantly reduced after treatment. Tear concentrations of IL-5, IL-7, IL-12, IL-13, G-CSF, and MIP-1β were also reduced after treatment, but the reductions were not statistically significant (P > 0.01).
This study demonstrates that the clinical manifestations of ocular demodecosis are considerably improved and the tear levels of IL-1β and IL-17 are significantly decreased after eradication of Demodex. Previously, several studies have reported that Demodex may cause ocular surface inflammation and pathological features and that lid scrubbing using tea tree oil was an effective method for eradicating Demodex (4, 8, 9). Similarly, we observed chronic refractory pathological ocular surface appearances (such as large corneal nodular opacity, corneal vascularization, severe corneal erosion and infiltration, and conjunctival inflammatory reactions) in patients with ocular demodecosis, and we confirmed the clinical improvement of ocular manifestation after the treatment of demodecosis in this study.
All patients in our study had not only intractable ocular surface manifestations but prior inflammatory ocular surface disorders including herpetic stromal keratitis, atopic keratoconjunctivitis, allergic conjunctivitis, and severe dry eye. Recommended conventional treatment was not successful but the eradication of Demodex improved the pathological ocular surface manifestations. This suggests that Demodex plays a pathological role or at least an aggravating role in inflammatory ocular surface disorders, especially immunological disorders. Type III or type IV hypersensitivity immune response is an important pathological mechanism in herpetic stromal keratitis and type I hypersensitivity immune responses is a primary mechanism in atopic keratoconjunctivitis and allergic conjunctivitis. So we believe that the pathogenesis of Demodex may be associated with hypersensitive immune responses on the ocular surface.
The levels of tear cytokines can be used to indicate inflammatory or immunological responses on the ocular surface. In this study, we observed that concentrations of IL-1β and IL-17 were reduced after treatment with TTO lid scrubs, and that these reductions were statistically significant.
IL-1 is a potent inducer of other inflammatory cytokines, including IL-6, IL-8, TNF-α, and granulocyte-macrophage colony-stimulating factor (GMCSF), and stimulates production of collagenase and matrix metalloproteinase (MMP) enzymes by epithelial cells, keratocytes, and inflammatory cells (12-14). IL-1 induces the destruction of extracellular matrix and renders inflammatory damage on the ocular surface. Clinically, there have been reports that IL-1β is present at increased levels in tears in patients suffering inflammatory ocular surface disorders such as dry eye, Sjögren syndrome, and keratoconjunctivitis sicca (15). It is therefore reasonable to conclude that the improvements of chronic refractory corneal epithelial erosions and ocular surface inflammatory signs observed after eradicating Demodex reflect reductions in IL-1. However, it is difficult to specify the particular inflammatory process in ocular demodecosis because IL-1 is a cytokine involved in various immune responses and inflammatory processes.
IL-17 is an important cytokine in inflammatory and autoimmune conditions (16). It is secreted mainly by activated helper T cells and enhances the generation, activation, and migration of neutrophils through the induction of proinflammatory cytokines (17, 18). T cells secreting IL-17, named Th17 cells, are now considered developmentally distinct from Th1 and Th2 cells and are thought to play a key role in autoimmune reactions (19, 20). IL-17 activates T cells and other immune cells to produce a variety of cytokines and cell adhesion molecules not only in the early neutrophil-mediated inflammatory response, but also in the induction of both Th1-type and Th2-type immune responses (21, 22). Synergy with other inflammatory cytokines such as IL-1β, tumor necrosis factor (TNF)-α, and IFN-γ leads to up-regulation of gene expression and progression and amplification of local inflammation (23, 24). Levels of IL-17 are significantly increased in autoimmune diseases including rheumatoid arthritis synovium, asthmatic airways, during allograft rejection, and in other chronic inflammatory diseases including multiple sclerosis and psoriasis (25-28). In this study, the reduction in tear levels of IL-17 after Demodex eradication suggests that ocular demodecosis is associated with elevated cell mediated immune conditions, especially the Th17 cell immune response. IL-17 is also a potent inducer of angiogenic chemokines such as vascular endothelial growth factor-A (VEGF-A) from a number of cells, including keratinocytes, fibroblasts and epithelial cells (29, 30). It is reasonable to explain that decreased levels of tear IL-17 following Demodex eradication contribute to the improvement of abnormal corneal vascularization.
There are some limitations in this study including the small number of subjects, the possible anti-inflammatory effect of tea tree oil, and the possibility of other inflammatory factors associated with Demodex, such as bacterial distribution. Future studies should include normal control subjects or blepharitis control subjects without Demodex, a larger number of subjects, an assessment of the anti-inflammatory effects of tea tree oil and the bacterial distribution in ocular demodecosis, and a wider analysis of cytokines associated with the Th17 immune response including IL-6, IL-21, IL-22, IL-23, and transforming growth factor beta (TGF-β). With further study, we may be able to confirm the pathogenic mechanism of Demodex on the ocular surface.
In summary, we examined chronic refractory inflammatory pathological ocular surface manifestations in patients with ocular demodecosis and verified that lid scrubbing with tea tree oil was an effective method to eradicate Demodex leading to clinical improvement. We also noted that tear concentrations of IL-1β and IL-17 were significantly decreased after the eradication of Demodex in the patients. Therefore, we believe that Demodex plays an aggravating role in inflammatory ocular surface disorders and that the treatment of Demodex induces the recovery of pathologic clinical manifestations.
Figures and Tables
Fig. 1
Representative microscopic photographs of Demodex folliculorum in patients with ocular demodecosis. Two D. folliculorum with an eyelash (A, arrows) and one free D. folliculorum are found in epilated eyelashes of patients.

Fig. 2
Clinical features of patients with ocular demodecosis (A, case 4; B, case 5; C, case 9; D, case 1; E, case 3; F, case 7; G, case 2; H, case 6; I, case 8). Large corneal nodular opacity (A-C), refractory corneal erosion and infiltration (D-F), peripheral corneal vascularization (C, G, H), and palpebral conjunctival papillary hypertrophy (I) are observed in patients with ocular demodecosis.
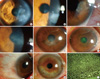
Fig. 3
Clinical appearance before and after lid scrubbing treatment with tea tree oil in patients with ocular demodecosis. (A and D, case 4; B and E, case 9; C and F, case 7). There are corneal nodular opacities (A, B), bulbar conjunctival injections (A, B) and central corneal infiltration (C) before the treatment. However, four weeks after the treatment, corneal opacities are markedly faded and conjunctival injections had resolved (D, E). Central corneal infiltration had also resolved (F).
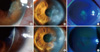
Table 1
Demographic and clinical features of patients with Demodex blepharitis and chronic ocular surface disorders

AUTHOR SUMMARY
Clinical and Immunological Responses in Ocular Demodecosis
Jae Hoon Kim, Yeoun Sook Chun and Jae Chan Kim
Demodex is thought to be associated with blepharitis, allergic conjunctivitis, and pathological corneal features on the ocular surface. However, the pathogenesis of Demodex on the ocular surface remains unclear. We evaluated clinical manifestations and tear cytokine level changes following the eradication of Demodex on the inflammatory ocular surface disorders. After a course of treatment of lid scrubbing with tea tree oil in patients with ocular demodecosis, Demodex was nearly eradicated, and there was substantial clinical improvement and tear concentrations of IL-1β and IL-17 were significantly reduced in all patients. Therefore, we believe that Demodex plays an imporatant aggravating role in inflammatory ocular surface disorders.
References
1. Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002. 82:3–6.
2. English FP, Nutting WB. Demodicosis of ophthalmic concern. Am J Ophthalmol. 1981. 91:362–372.
3. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophthalmol Soc. 1967. 65:361–392.
4. Kheirkhah A, Casas V, Li W, Raju VK, Tseng SC. Corneal manifestations of ocular demodex infestation. Am J Ophthalmol. 2007. 143:743–749.
5. Rodríguez AE, Ferrer C, Alió JL. Chronic blepharitis and Demodex. Arch Soc Esp Oftalmol. 2005. 80:635–642.
6. Kheirkhah A, Blanco G, Casas V, Tseng SC. Fluorescein dye improves microscopic evaluation and counting of demodex in blepharitis with cylindrical dandruff. Cornea. 2007. 26:697–700.
7. Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005. 46:3089–3094.
8. Gao YY, Di Pascuale MA, Li W, Baradaran-Rafii A, Elizondo A, Kuo CL, Raju VK, Tseng SC. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005. 89:1468–1473.
9. Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007. 26:136–143.
10. Jones DT, Monroy D, Pflugfelder SC. A novel method of tear collection: comparison of glass capillary micropipettes with porous polyester rods. Cornea. 1997. 16:450–458.
11. Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O'Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006. 25:900–907.
12. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991. 5:2145–2154.
13. Strissel KJ, Girard MT, West-Mays JA, Rinehart WB, Cook JR, Brinckerhoff CE, Fini ME. Role of serum amyloid A as an intermediate in the IL-1 and PMA-stimulated signaling pathways regulating expression of rabbit fibroblast collagenase. Exp Cell Res. 1997. 237:275–287.
14. Strissel KJ, Rinehart WB, Fini ME. Regulation of paracrine cytokine balance controlling collagenase synthesis by corneal cells. Invest Ophthalmol Vis Sci. 1997. 38:546–552.
15. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999. 19:201–211.
16. McGeachy MJ, Anderton SM. Cytokines in the induction and resolution of experimental autoimmune encephalomyelitis. Cytokine. 2005. 32:81–84.
17. Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996. 183:2593–2603.
18. Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998. 160:3513–3521.
19. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005. 6:1123–1132.
20. Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007. 19:281–286.
21. Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guérin infection. J Immunol. 2007. 178:3786–3796.
22. Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002. 17:375–387.
23. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004. 279:2559–2567.
24. Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999. 162:494–502.
25. Chabaud M, Lubberts E, Joosten L, van Den Berg W, Miossec P. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001. 3:168–177.
26. Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999. 162:577–584.
27. Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998. 111:645–649.
28. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001. 108:430–438.
29. Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001. 167:4137–4140.
30. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003. 101:2620–2627.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download