Abstract
We have hypothesized that non-dipper status and left ventricular hypertrophy (LVH) are associated with the development of chronic kidney disease (CKD) in non-diabetic hypertensive patients. This study included 102 patients with an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2. Ambulatory blood pressure monitoring and echocardiography were performed at the beginning of the study, and the serum creatinine levels were followed. During the average follow-up period of 51 months, CKD developed in 11 patients. There was a significant difference in the incidence of CKD between dippers and non-dippers (5.0% vs 19.0%, P < 0.05). Compared to patients without CKD, patients with incident CKD had a higher urine albumin/creatinine ratio (52.3 ± 58.6 mg/g vs 17.8 ± 29.3 mg/g, P < 0.01), non-dipper status (72.7% vs 37.4%, P < 0.05), the presence of LVH (27.3% vs 5.5%, P < 0.05), and a lower serum HDL-cholesterol level (41.7 ± 8.3 mg/dL vs 50.4 ± 12.4 mg/dL, P < 0.05). Based on multivariate Cox regression analysis, non-dipper status and the presence of LVH were independent predictors of incident CKD. These findings suggest that non-dipper status and LVH may be the therapeutic targets for preventing the development of CKD in non-diabetic hypertensive patients.
Chronic kidney disease (CKD) is emerging as a worldwide public health problem because of the rising prevalence and incidence. Although early detection and therapeutic interventions may slow or prevent the progression toward renal failure and the associated complications, CKD is usually under-diagnosed and under-treated, resulting in unfavorable outcomes (1, 2). One of the reasons is thought to be an under-recognition of the earlier stages of CKD and risk factors for CKD.
Among the commonly considered risk factors for the development of CKD such as age, diabetes, hypertension, vascular disease, and ethnicity (3), diabetes is known to be the strongest determinant (4). In non-diabetic patients, however, it appears that predictors of incident CKD have not been well elucidated.
There are tremendous evidences for proving that the night-time blood pressure (BP) is the more powerful predictor in cardiovascular morbidity and mortality than conventional office and ambulatory daytime BP (5). In healthy subjects, there is a diurnal variation in BP which decreases by 10%-20% during the nighttime compared to the daytime. Patients without a nocturnal BP decline ≥ 10% of daytime values are referred to as non-dippers (6).
The rate of decline in the glomerular filtration rate (GFR) is faster in hypertensive patients with blunted diurnal BP variation (7). In addition, the non-dipping phenomenon in end-stage renal disease (ESRD) patients is closely related to a high incidence of cardiovascular disease and a poor long-term survival (8). Therefore, it has become important to observe 24-hr ambulatory blood pressure monitoring (ABPM) patterns, including blunting or loss of diurnal variation, in hypertensive and CKD patients.
Left ventricular hypertrophy (LVH) is regarded as a common target organ damage of hypertension (9). Moreover, LVH is associated with an increased incidence of cardiovascular events (10). In CKD patients, LVH is more prevalent and more severe than in hypertensive patients with normal renal function (11), and accompanied by increased cardiovascular mortality, even in patients with mild renal dysfunction (12). Furthermore, it was recently reported that LVH is a predictor of subsequent renal dysfunction in men with high cardiovascular risk (13).
However, there is little evidence that the non-dipping pattern of nocturnal BP or LVH is associated with the development of CKD in hypertensive patients with preserved renal function. In the present study, we determined whether or not non-dipper status and LVH are predictors of incident CKD in non-diabetic hypertensive patients treated with antihypertensive medications.
We conducted the analysis using a retrospective cohort. This study included patients with essential hypertension recruited for a cross-sectional study, the precise methods have been described elsewhere (14). Briefly, participants were between 30 and 80 yr of age with essential hypertension and treated with antihypertensive drugs for at least 1 yr. The exclusion criteria were a history of diabetes mellitus or a fasting blood glucose > 126 mg/dL at the time of enrollment to the study, any previous cardiovascular events, a history of liver cirrhosis, inflammatory disease, and a random urine albumin/creatinine ratio (ACR) of ≥ 300 mg/g, and use of various confounding medications. The patients were followed and antihypertensive medications were individualized to each subject, and were changed ad libitum on the basis of the clinician's decision during the follow-up period. In this cohort, we selected participants with an estimated GFR (eGFR) which was > 60 mL/min per 1.73 m2 at the time of enrollment, as determined by the abbreviated Modification of Diet in Renal Diseases (MDRD) formula (15), and in whom serum creatinine levels were followed ≥ 3 times until the time of the analysis. We finally enrolled 102 subjects in the current study. Incident CKD was defined as an eGFR decreased to < 60 mL/min/1.73 m2 persisting for at least 3 months.
At the beginning of the study, patients underwent a complete physical examination, demographic and laboratory assessments, 24-hr ABPM, and a baseline electrocardiogram. The following laboratory parameters were performed: blood chemistries; high sensitivity C-reactive protein (hsCRP); and random urine analysis for urine ACR.
Office BP was measured in the outpatient clinic, using a sphygmomanometer after at least 5 min of rest in a sitting position. Two blood pressures were measured at least 5 min apart and the mean BP was used for analysis.
Twenty-four-hour ABPM was performed using a Takeda TM-2421 (A&D Medical, Tokyo, Japan). The daytime BP recording was done every 30 min from 7 AM to 11 PM and the nighttime BP recording was done every 30 min from 11 PM to 7 AM.
The recordings for 24 hr were then analyzed to obtain daytime and nocturnal average systolic BP (SBP) and diastolic BP (DBP). Nocturnal BP dipping was defined as ≥ 10% decrease in nocturnal SBP and DBP compared to the average daytime SBP and DBP. Patients with < 10% decrease or increase more than the daytime BP in the nighttime BP were considered to be non-dippers in this study. Nocturnal hypertension was defined as an average nocturnal BP > 125/75 mmHg.
Echocardiography was performed with an ultrasound system (Vivid 7 GE Vingmed, Horten, Norway) with a 2.5 MHz transducer and interpreted by two experienced readers blinded to the clinical information. Standard two-dimensional parameters were measured with the patient in the left lateral position. The left ventricular (LV) ejection fraction was calculated. To evaluate LV filling pressures, the ratio mitral inflow peak velocity (E)/early diastolic tissue velocity of the mitral annulus (E') was calculated. LV mass was determined by the method of Devereux et al. (16), with the LV mass index calculated by dividing the LV mass by the body surface area. Men with a LV mass index > 125 g/m2 and women with a LV mass index > 100 g/m2 were considered to have LVH.
Statistical analysis was performed with SPSS 16.0 (SPSS Inc, Chicago, IL, USA). The continuous variables are presented as the mean ± standard deviation, or patient number (%). The differences between any two groups were evaluated by a chi-square test for categorical data and Student's t-test for continuous variables. To investigate the predictors of the development of CKD, Cox proportional hazard analysis was performed. Any difference was considered significant when a P value was < 0.05.
The baseline characteristics and BP measurements of the study populations are shown in Table 1. The mean age of the patients was 56.0 ± 10.4 yr and the mean duration of hypertension was 93.6 ± 36.1 months. Based on the 24-hr ABPM data, the mean fulltime SBP and DBP were 125.8 ± 14.0 mmHg and 78.6 ± 9.2 mmHg, respectively. The mean initial eGFR was 81.4 ± 16.1 mL/min/1.73 m2, and the mean initial urine ACR was 21.1 ± 34.2 mg/g.
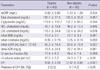
When the study population was divided into dippers and non-dippers based on the 24-hr ABPM results, 42 of 102 patients were classified as non-dippers. There were no significant differences in age, body mass index (BMI), and the duration of hypertension between the two groups. However, the non-dippers demonstrated lower proportion of males (46.7% vs 26.2%, P < 0.05) and higher office SBP (130.2 ± 17.7 mmHg vs 139.0 ± 22.9 mmHg, P < 0.05) compared to the dippers. The data from the 24-hr ABPM were not significantly different in the mean fulltime BP and mean daytime BP between the two groups, while the mean nighttime BP (SBP, 111.5 ± 11.1 mmHg vs 123.7 ± 17.8 mmHg, P < 0.001; DBP, 69.5 ± 7.7 mmHg vs 75.0 ± 9.4 mmHg, P < 0.01) and pulse pressure (42.0 ± 7.4 mmHg vs 48.7 ± 13.6 mmHg, P < 0.01) were significantly higher, and the nocturnal hypertension was more prevalent in the non-dippers (25.0% vs 61.9%, P < 0.001; Table 2). Between the two groups, there were no significant differences in laboratory findings including initial eGFR and urine ACR. In addition, there were no significant differences in the LV ejection fraction, whereas parameters which indicate LV diastolic dysfunction such as left atrial (LA) volume index (20.5 ± 5.2 mL/m2 vs 24.3 ± 7.3 mL/m2, P < 0.01) and E/E' (9.65 ± 2.29 vs 12.42 ± 3.88, P < 0.001), and the presence of LVH (3.3% vs 14.3%, P < 0.05) were higher in the non-dippers (Table 3).
During the average follow-up period of 51 months (range, 13-64 months), there were no differences in BUN, creatinine, and eGFR between the dippers and non-dippers. Although the annual change rate of the eGFR showed no significant differences (-0.20 ± 6.59 mL/min/1.73 m2/year vs -1.36 ± 4.68 mL/min/1.73 m2/yr, P = 0.303), the incident CKD patients whose eGFR was reduced to < 60 mL/min per 1.73 m2 and persisted for at least 3 months were more frequently observed in the non-dippers (5.0% vs 19.0%, P < 0.05).
The decline rate of the eGFR was significantly higher in patients with incident CKD than in patients without CKD (-4.38 ± 4.67 mL/min/1.73 m2/yr vs -0.23 ± 5.88 mL/min/1.73 m2/yr, P < 0.05). There were no significant differences in age, gender, BMI, duration of hypertension, duration of follow-up, office BP, and all parameters on 24-hr ABPM between patients with newly developed CKD and without CKD. However, the proportion of non-dippers was significantly higher in patients with CKD than in patients without CKD (72.7% vs 37.4%, P < 0.05). Patients with newly developed CKD revealed a lower HDL-cholesterol (41.7 ± 8.3 mg/dL vs 50.4 ± 12.4 mg/dL, P < 0.05) and higher urine ACR (52.3 ± 58.6 mg/g vs 17.8 ± 29.3 mg/g, P < 0.01) and a higher proportion of patients with LVH (27.3% vs 5.5%, P < 0.05) compared with patients without CKD.
Univariate Cox regression analysis revealed that the duration of hypertension (hazard ratio [HR], 1.09; 95% confidence interval [CI], 1.00-1.18), non-dipper status (HR 7.90; 95% CI, 2.02-30.91), E/E' (HR 1.27; 95% CI, 1.08-1.48), and LVH (HR 6.85; 95% CI, 1.81-25.93) were significant for CKD development. However, age, gender, BMI, smoking history, office BP, mean BP measured by 24-hr ABPM, initial eGFR, urine ACR, LV ejection fraction, and LA volume index were not significantly related to the development of CKD. Further adjustments revealed that non-dipper status and LVH remained robust as significant predictors of incident CKD. Non-dipper status (HR 30.76; 95% CI, 1.75-542.12) and LVH (HR 175.97; 95% CI, 4.03-7679.90) were independent predictors of incident CKD when adjustments were made for age, gender, office BP, duration of hypertension, initial eGFR, urine ACR and E/E' (Table 4).
In the present study, we demonstrated that patients with newly developed CKD, whose eGFR was reduced < 60 mL/min per 1.73 m2 and persisted for more than 3 months, were significantly more frequent in the non-dippers on 24-hr ABPM in non-diabetic hypertensive patients. In addition, non-dipper status and LVH were predictors of incident CKD in non-diabetic hypertensive patients independent of office BP or mean fulltime/daytime BP.
In epidemiologic studies, age, gender, ethnicity, diabetes, hypertension, vascular diseases, obesity, smoking, low HDL-cholesterol level, and a mild reduction in GFR were important risk factors for new-onset CKD (3, 4). Because a considerable portion of participants in those epidemiologic studies had diabetes, metabolic syndrome, and other risk factors for vascular diseases, determinants for incident CKD in low-risk, non-diabetic hypertensive patients still remain for evaluation. In the present study, none of the participants had diabetes, macroalbuminuria and any form of vascular diseases. Therefore, the participants in this study were considered to have a low probability for the development of CKD.
Although it is generally agreed that hypertension exacerbates all forms of CKD and accelerates progression to ESRD, the epidemiologic evidence supporting mild-to-moderate essential hypertension as an initiator of kidney damage has been weak (17). Along these lines, it has been reported that only 'genetically susceptible' patients with hypertension will develop CKD (18). In the current study, the non-dipping pattern on 24-hr ABPM and LVH on echocardiography, which could be easily confirmed at the bedside, was identified as predictors of new-onset CKD.
The relationship between the non-dipping phenomenon and existing CKD has been previously reported. Non-dipping is significantly more prevalent in patients with underlying renal disease, and the prevalence increases with worsening renal function in up to 80% of ESRD patients (19). The nocturnal fall in BP is attenuated in patients with polycystic kidney disease or IgA nephropathy (20, 21). In diabetic patients, a blunted nocturnal fall in BP is closely associated with albuminuria (22). Furthermore, the non-dipping phenomenon is associated with a faster progression of renal insufficiency in pre-dialysis CKD patients (6), and an increased risk of total mortality and the composite end point of incident ESRD and death in a cohort study of veterans with CKD (23).
LVH was a predictor for subsequent renal dysfunction in high-risk hypertensive patients in a recent epidemiologic study (13), as well as an epiphenomenon in hypertensive patients and a risk factor for cardiovascular events. A cross-sectional analysis revealed that renal dysfunction defined as creatinine clearance < 60 mL/min/1.73 m2 or microalbuminuria was independently related to the presence of LVH (24). In accordance with previous studies, LVH was a potent factor for predicting incident CKD in this study.
Increased LV mass is often accompanied by diastolic dysfunction and thus the diagnosis of diastolic heart failure can be made on the basis of LVH, clinical evidence of heart failure, and a normal LV ejection fraction (25). The ratio of mitral velocity to early diastolic velocity of the mitral annulus (E/E') reflects diastolic function (26). In the present study, E/E' was also significantly correlated with the presence of LVH (data not shown), and E/E' was a potential predictor for incident CKD based on univariate analysis, but not statistically significant on multivariate Cox regression analysis. It is likely because the subjects had a low prevalence of diastolic heart failure with symptoms and E/E' > 15.
In addition, dyslipidemia is thought to be a risk factor for the development of CKD. Subjects with an elevated ratio of LDL- to HDL-cholesterol had a faster decline, and both the lipoprotein ratio and HDL-cholesterol level were significant risk factors (27). In the Atherosclerosis Risk in Communities (ARIC) study, individuals with higher triglycerides and lower HDL-cholesterol at baseline were independent risk factors for a rise in creatinine (28). In the current study, we also showed that patients with new-onset CKD had lower HDL-cholesterol; however, the relationship did not reach statistical significance in a Cox regression model.
In contrast to previous results (3), age, BMI, smoking, and eGFR were not significant predictors of incident CKD in the present study. Although, the initial eGFR was significantly lower in patients with new-onset CKD than in patients without CKD, it did not reach statistical significance based on Cox regression analysis. In addition, the rate of decline in eGFR was also higher in incident CKD patients compared with patients without CKD. Therefore, patients with incident CKD might have characteristics other than a lower initial eGFR.
There were some limitations to the present study. Only one assessment of 24-hr ABPM was performed in all patients at baseline. Despite the difference in baseline renal function of the participants, the consistency in circadian BP variation was reported to be poor in CKD stages 3-5 and single measurements of 24-hr ABPM were probably inadequate for the evaluation of dipping status (29). In addition, because it was a retrospective study, antihypertensive medications during the follow-up period were not equally controlled. Furthermore, uniform intervention of use in antihypertensive medications was not performed; therefore we could not evaluate the effect of different usage of various antihypertensives. Finally, we could not compare other outcomes according to dipper status, such as cardiovascular morbidity and mortality, with the exception of renal outcomes, due to the relatively small size and short follow-up periods.
In conclusion, although the mechanism for the relationship between the non-dipping phenomenon and decline in renal function is not clearly understood in the present study, a non-dipping pattern on 24-hr ABPM and LVH are independent predictors of incident CKD in hypertensive patients without diabetes and other vascular diseases. These findings suggest that non-dipper status and LVH may be the therapeutic targets for preventing the development of CKD in non-diabetic hypertensive patients.
Figures and Tables
Table 2
Comparisons of initial demographic and clinical characteristics between dippers and non-dippers
AUTHOR SUMMARY
Non-Dipper Status and Left Ventricular Hypertrophy as Predictors of Incident Chronic Kidney Disease
Hye Rim An, Sungha Park, Tae-Hyun Yoo, Shin-Wook Kang, Jung-Hwa Ryu, Yong Kyu Lee, Mina Yu, Dong-Ryeol Ryu, Seung Jung Kim, Duk-Hee Kang and Kyu Bok Choi
Although hypertension exacerbates all forms of chronic kidney disease (CKD), it is still unclear whether mild-to-moderate essential hypertension is an initiator of kidney damage. In the present study, we have identified that a non-dipping pattern of 24-hr blood pressure and left ventricular hypertrophy (LVH) are independent predictors of incident CKD in "low-risk" hypertensive patients without diabetes and other vascular diseases. These findings suggest that non-dipper status and LVH may be the therapeutic targets for preventing the development of CKD.
References
1. Pereira BJ. Optimization of pre-ESRD care: the key to improved dialysis outcomes. Kidney Int. 2000. 57:351–365.
2. Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001. 12:1713–1720.
3. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004. 291:844–850.
4. Hanratty R, Chonchol M, Miriam Dickinson L, Beaty BL, Estacio RO, Mackenzie TD, Hurley LP, Linas SL, Steiner JF, Havranek EP. Incident chronic kidney disease and the rate of kidney function decline in individuals with hypertension. Nephrol Dial Transplant. 2010. 25:801–807.
5. Yoon SY, Pyun WB. Clinical significance of nighttime blood pressure. Korean J Med. 2011. 80:31–35.
6. Timio M, Venanzi S, Lolli S, Lippi G, Verdura C, Monarca C, Guerrini E. "Non-dipper" hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995. 43:382–387.
7. Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006. 166:846–852.
8. Liu M, Takahashi H, Morita Y, Maruyama S, Mizuno M, Yuzawa Y, Watanabe M, Toriyama T, Kawahara H, Matsuo S. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003. 18:563–569.
9. Elias MF, Sullivan LM, Elias PK, D'Agostino RB Sr, Wolf PA, Seshadri S, Au R, Benjamin EJ, Vasan RS. Left ventricular mass, blood pressure, and lowered cognitive performance in the Framingham offspring. Hypertension. 2007. 49:439–445.
10. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990. 322:1561–1566.
11. Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996. 27:347–354.
12. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005. 293:1737–1745.
13. Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, Doumas M, Stefanadis C, Papademetriou V. Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypertens. 2010. 28:2299–2308.
14. Seo HS, Kang TS, Park S, Choi EY, Ko YG, Choi D, Ha J, Rim SJ, Chung N. Non-dippers are associated with adverse cardiac remodeling and dysfunction (R1). Int J Cardiol. 2006. 112:171–177.
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999. 130:461–470.
16. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986. 57:450–458.
17. Freedman BI, Sedor JR. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol. 2008. 19:2047–2051.
18. Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008. 40:1185–1192.
19. Farmer CK, Goldsmith DJ, Cox J, Dallyn P, Kingswood JC, Sharpstone P. An investigation of the effect of advancing uraemia, renal replacement therapy and renal transplantation on blood pressure diurnal variability. Nephrol Dial Transplant. 1997. 12:2301–2307.
20. Li Kam Wa TC, Macnicol AM, Watson ML. Ambulatory blood pressure in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1997. 12:2075–2080.
21. Csiky B, Kovács T, Wágner L, Vass T, Nagy J. Ambulatory blood pressure monitoring and progression in patients with IgA nephropathy. Nephrol Dial Transplant. 1999. 14:86–90.
22. Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002. 347:797–805.
23. Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006. 69:1175–1180.
24. Smilde TD, Asselbergs FW, Hillege HL, Voors AA, Kors JA, Gansevoort RT, van Gilst WH, de Jong PE, Van Veldhuisen DJ. Mild renal dysfunction is associated with electrocardiographic left ventricular hypertrophy. Am J Hypertens. 2005. 18:342–347.
25. Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med. 2004. 351:1097–1105.
26. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000. 102:1788–1794.
27. Mänttäri M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995. 26:670–675.
28. Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000. 58:293–301.
29. Elung-Jensen T, Strandgaard S, Kamper AL. Longitudinal observations on circadian blood pressure variation in chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2008. 23:2873–2878.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download