Abstract
The interleukin-33 (IL-33)/ST2 pathway has emerged as an intercellular signaling system that participates in antigen-allergen response, autoimmunity and fibrosis. It has been suggested that IL-33/ST2 signaling has been involved in the pathogenesis of rheumatoid arthritis (RA), because IL-33 and its receptor have been specifically mapped to RA synovium. The aim of this study was to determine the levels of IL-33 and sST2 in sera and synovial fluids in patients with RA. The serum level of IL-33 was significantly higher in patients with RA (294.9 ± 464.0 pg/mL) than in healthy controls (96.0 ± 236.9 pg/mL, P = 0.002). The synovial fluid level of IL-33 was significantly higher in RA patients than in osteoarthritis patients. The level of serum sST2 was higher in RA patients than in healthy controls (P = 0.042). A significant relationship was found between the levels of IL-33 and IL-1β (r = 0.311, P = 0.005), and IL-33 and IL-6 (r = 0.264, P = 0.017) in 81 RA patients. The levels of IL-33, sST2 and C-reactive protein decreased after conventional disease-modifying antirheumatic drugs treatment in 10 patients with treatment-naïve RA. Conclusively, IL-33 is involved in the pathogenesis of RA and may reflect the degree of inflammation in patients with RA.
Interleukin-33 (IL-33) is a member of the IL-1 family, which includes IL-1α, IL-1β, IL-1 receptor antagonist, and IL-18. In humans, IL-33 is found predominantly in the skin, lung, adipocytes, and synovial fibroblasts (1, 2). Similar to IL-1β and IL-18, IL-33 is considered to be produced intracellularly as pro-IL-33, which does not contain a signal peptide sequence for secretion, and then released extracellularly as mature IL-33 after cleavage. Mature IL-33 has been reported to mediate its biologic effects via T1/ST2 binding by activating NF-κB and MAP kinase (3).
IL-33 binds to its receptor complex, which comprises ST2L and IL-1 receptor accessory protein on eosinophils, basophils, mast cells, natural killer (NK) cells, Th2 lymphocytes, and invariant natural killer T (iNKT) cells (4). IL-33 enhances adhesion and CD11b expression in human eosinophils and basophils (5). IL-33 induces the production of IL-6, IL-1β, tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1, and prostaglandin D2 production in bone marrow-derived mast cells (6). It also increases the production of IL-5 and IL-13 by polarized Th2 cells (3) and interferon-γ (IFN-γ) production by both iNKT and NK cells (7).
ST2 is a member of the IL1R/TLR superfamily. There are three isoforms of ST2 in humans, which are produced by differential splicing of a single transcript: sST2, ST2L, and ST2V. soluble(s) ST2, the secreted soluble form, is expressed in embryonic tissues, mammary tumors, and fibroblasts (8). The expression of ST2L, a membrane-anchored long form, is restricted to the surface of Th2 cells and mast cells (3). ST2V is expressed mainly in gastrointestinal organs such as the stomach, large and small intestine, and spleen (9). Whereas ST2L mediates the effect of IL-33 on Th2-dependent inflammatory processes, sST2 acts as a decoy receptor that prevents the interaction of ST2L with IL-33. sST2 has been implicated in the attenuation of the Th2-induced inflammatory response.
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by synovitis, bone destruction with pannus formation, and degradation of articular cartilage. It has been suggested that IL-33/ST2 signaling is involved in the pathogenesis of a wide array of diseases including asthma, fibroproliferative disease, and autoimmune diseases such as systemic lupus erythematosus (SLE), progressive systemic sclerosis, Wegener's granulomatosis, and Behçet's disease. Recent studies have suggested that IL-33/ST2 is involved in the pathogenesis of RA. IL-33 mRNA is expressed in the RA synovial membrane, predominantly in fibroblast-like synoviocytes. IL-33 administration exacerbates collagen-induced arthritis (CIA) in mice, and disease severity is reduced in mice administered with sST2-Fc fusion protein or anti-IL-33 monoclonal antibody (10). Injection of IL-33 into a joint induces localized mechanical damage or articular hypernociception, which can be inhibited by sST2 treatment in mice (11).
In patients with RA, the level of sST2 is elevated in the serum (12). However, little is known about the levels of IL-33 in RA patients. In this study, we measured the levels of IL-33 and sST2 in sera and synovial fluids of RA patients and examined whether the levels of IL-33 or sST2 could reflect the degree of inflammation.
This was a multicenter study conducted by Catholic University and Soonchunhyang University in Korea from May 2008 to January 2009. Blood samples were drawn from 81 patients with RA, 18 patients with osteoarthritis (OA), and 50 healthy subjects. Synovial fluids were obtained from 16 RA patients and 10 OA patients, and IL-33 and sST2 levels were measured. RA was diagnosed based on the American College of Rheumatology 1987 criteria (13). When the patients had never been received any kind of disease-modifying antirheumatic drugs (DMARDs), it was defined as "treatment-naïve". On the other hand, treated RA was defined when the patients had been administered any kind of DMARDs. None of all the patients that were enrolled in this study had ever received the biological agents for the treatment of RA. The serum levels of IL-33, sST2, and C-reactive protein (CRP) were measured before and after treatment with DMARDs in 10 treatment-naïve RA patients.
The IL-33, IL-1β, and IL-6 concentrations in diluted serum and synovial fluid were measured by sandwich ELISA. Briefly, 4 µg/mL of monoclonal capture antibody (R&D Systems, Minneapolis, MN, USA) was added to a 96-well plate (Nunc, Rochester, NY, USA) and the plate was incubated for 2 hr at room temperature. The plate was then incubated in blocking solution comprising phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.05% Tween 20 for 2 hr at room temperature. All sera and synovial fluid samples were diluted 1:2 by using PBS containing 1% bovine serum albumin and 0.05% Tween 20. And standard curves were also diluted with PBS containing 1% bovine serum albumin and 0.05% Tween 20.
The test samples and standard recombinant IL-33, IL-1β, and IL-6 (R&D Systems) were added to the plates, and the plate was incubated for 2 hr at room temperature. The plate was washed four times with PBS containing Tween 20, 200 ng/mL of biotinylated detection monoclonal antibodies (R&D Systems) was added, and the plate was incubated for 2 hr at room temperature. The plate was washed, streptavidin-alkaline-phosphatase (Sigma-Aldrich, St Louis, MO, USA; diluted 1:2,000) was added, and the reaction was allowed to proceed for 2 hr at room temperature. The plate was washed four times, and 1 mg/mL of p-nitrophenyl phosphate dissolved in diethanolamine (both from Sigma-Aldrich) was added to induce the color reaction, which was stopped by adding 50 µL of 1 N NaOH. The optical density at 405 nm was measured on an automated microplate reader (VERSAmax, Molecular Devices, Palo Alto, CA, USA). A standard curve was drawn by plotting optical density versus the log of the concentrations of IL-33, IL-1β, and IL-6. The ST2/IL-1R4 level was measured using the DuoSet ELISA kit (R&D Systems) following the manufacturer's protocol.
Biopsy specimens from the synovium of patients with RA and OA were surgically removed and placed in 4% paraformaldehyde solution overnight at 4℃. The fixed tissues were embedded in paraffin and sectioned at 7-µm thickness. Paraffin-embedded slides were deparaffinized by immersion in xylene and then dehydrated in ethanol. Sections were depleted of endogenous peroxidase activity by adding methanolic H2O2. Immunohistochemical staining was performed using a Vectastain ABC kit (Vector, Peterborough, UK). After overnight incubation at 4℃ with anti-IL-33 and anti-ST2/IL-1 (R&D Systems), samples were incubated for 20 min with the secondary antibodies, biotinylated anti-goat IgG and then incubated with streptavidin-peroxidase complex for 1 hr followed by incubation with 3,3'-diaminobenzidine (Dako, Glostrup, Denmark) for 5 min. The sections were counterstained with Mayer's hematoxylin, and the samples were photographed with an Olympus (Tokyo, Japan) photomicroscope.
All data are expressed as the mean ± standard deviation (SD). Continuous variables from the study were analyzed by the ANOVA and/or the Student's t test with a parametric distribution or the Mann-Whitney U test with a nonparametric distribution. Categorical data were compared using the chi-square test. Correlations between each variable were evaluated using the Spearman rank-order correlation test. All statistical analyses were performed using SPSS software (Chicago, IL, USA). Differences of P < 0.05 were considered significant.
The clinical and serological characteristics of the patients are described in Table 1. None of the patients with RA had administered any kind of anti-TNF therapy. Other medications in Table 1 include sulfasalazine, leflunomide and cyclosporine. In the patients with OA, 7 out of 18 were taken NSAIDs only and the rest were not administered any kind of medications.
As shown in Fig. 1A, the mean value of IL-33 was significantly higher in sera of RA patients than those of healthy controls (294.9 ± 464.0 and 95.9 ± 236.9 pg/mL, respectively, P = 0.002). The serum levels of IL-33 were significantly higher in patients with OA (310.3 ± 462.6 pg/mL) than controls (P = 0.015). The sST2 serum concentration was significantly higher in patients with RA (767.1 ± 1,059.5 pg/mL) than in healthy controls (455.4 ± 621.1 pg/mL, P = 0.042). In contrast to IL-33, the serum levels of sST2 did not differ between the patients with OA (330.4 ± 244.3 pg/mL) and healthy controls but that were significantly lower in patients with OA than RA.
The synovial fluid levels of IL-33 and sST2 were also measured in 16 samples of RA and 10 samples of OA. The mean level of IL-33 was 2,235.8 ± 5,035.4 pg/mL in the synovial fluids of RA, which was significantly higher than those of OA (424.8 ± 40.5 pg/mL, P = 0.015). However, the mean sST2 concentration of synovial fluid did not differ between the RA (2,508.3 ± 5,496.6 pg/mL) and OA (1,363.1 ± 2,156.7 pg/mL). These values are shown in Fig. 1B.
Similar to the synovial fluid levels of IL-33, the expressions of IL-33 and ST2L in RA synovium were greater than OA synovium (Fig. 2).
IL-6, IL-1β, and CRP levels were also measured in sera of patients with RA to confirm whether IL-33 is involved in the pathogenesis of RA and therefore reflects inflammatory status of the disease. Significant positive relationships were found between IL-33 and IL-1β levels (r = 0.311, P = 0.005) and IL-33 and IL-6 levels (r = 0.264, P = 0.017) in the 81 RA patients. The serum levels of sST2 also correlated significantly with both IL-6 and IL-1β levels in RA patients (Table 2). However, in the patients with OA, no significant correlation was observed between IL-33 and IL-1β or IL-6 levels, or between sST2 and IL-1β or IL-6 levels.
In 10 patients with treatment-naïve RA, the levels of IL-33, sST2, and CRP were compared before and after DMARDs therapy. The 9 patients out of them were administered more than 1 kind of DMARDs including methotrexate (MTX). MTX, hydroxychloroquine and sulfasalazine were administered to these patients. The remaining one patient received hydroxychloroquine and low dose steroid only because of the patient's refusal and low disease activity. Low dose glucocorticoids were administered to all of the patients. Fig. 3 shows the individual values of IL-33 and sST2 before and after treatment with DMARDs. The serum levels of both IL-33 and sST2 decreased after DMARDs therapy in the patients with RA (P = 0.034 and 0.012, respectively). As CRP is widely used to ascertain the degree of inflammatory response, we also measured the serum level of CRP. Along with IL-33 and sST2, CRP levels also decreased significantly after treatment with DMARDs in the patients with treatment-naïve RA. In addition, significant positive relationship (r = 0.827, P < 0.01) was found between the degree of IL-33 concentration decrease and that of CRP after treatment. In case of sST2, there was no relationship with the changes of CRP concentration (r = 0.547, P = 0.203).
In our study, the serum level of IL-33 was higher in the patients with RA as well as OA than healthy controls. And the IL-33 concentration was higher in RA synovial fluids than those of OA.
Palmer et al. (14) reported IL-33 staining in endothelial cells in both inflamed RA synovium and normal synovial tissue. While the resting synovial fibroblasts express little or no IL-33, its expression is markedly increased by TNF-α and IL-1β (15). Schmitz et al. (3) demonstrated that activated dendritic cells (DCs) and macrophages express low quantities of human IL-33 mRNA. The synovial fluids of RA patients and perivascular regions of RA synovium exhibit increased numbers of myeloid and plasmacytoid DCs (16). Macrophages are abundant in the inflamed synovial membrane and activation of macrophages in the inflamed synovial membrane correlates with the severity of rheumatoid arthritis (17). Therefore, synovial fibroblasts, endothelial cells, activated DCs, and macrophages may be the main source of IL-33 in synovial fluid from RA patients (3, 15).
Within our knowledge, there is only one study showing serum level of IL-33 in OA patients. In that report (18), serum level of IL-33 in OA patients is significantly lower than that of RA. Although OA is still generally considered a degenerative disorder, the development and progression of OA are now believed to involve inflammation even in the early stages of the disease. Secreted inflammatory factors such as proinflammatory cytokines are critical mediators of the disturbed metabolism and enhanced catabolism of joint tissue involved in OA. IL-1β, TNF and IL-6 seem to be the main proinflammatory cytokines involved in the pathophysiology of OA (19).
Fig. 1A showed elevated level of IL-33 in sera of both RA and OA patients comparing to those of healthy controls. It seems odds that serum level of IL-33 does not differ between the patients with RA and OA. Since the OA patients were older than RA in our study (61.6 ± 7.7 and 52.1 ± 13.3 yr, respectively), it is conceivable that serum level of IL-33 is affected by aging. So, we determined to compare the serum levels of IL-33 in younger OA patients (≤ 65 yr) with older (> 65 yr) to ascertain the impact of age on IL-33 level. The mean value of serum IL-33 was not different between the two groups (P = 0.167). And also, there was no difference in IL-33 concentration between OA patients and age, sex-matched RA patients (P = 0.313). Conclusively, the age factor did not seem to influence the level of circulating IL-33 in OA patients in this study. We think that these results could support the above belief in OA as an inflammatory disease.
Considering from another aspect, administration of glucocorticoids could reduce the serum level of IL-33 in the patients with RA. As showed in Table 1, over half of the RA patients were taken low dose of oral glucocorticoids. On the contrary, none of the OA patients was taken steroids. It is well known that glucocorticoids suppress many function in activated monocyte/macrophages, including the release of TNF-α. The use of oral glucocorticoids in RA patients might affect the serum concentration of TNF-α and consequently reduce the IL-33. This presumption could be supported by the recently published data that serum concentrations of IL-33 were reduced after anti-TNF therapy (18).
Although the mean concentration of serum IL-33 is not different to our surprise, the level of IL-33 in OA synovial fluids is significantly lower than that of RA. We assume that this discordant finding between the serum and synovial fluid might imply the different milieu between RA and OA joints.
One of our study limitations is lack of disease activity, such as Disease Activity Score 28 (DAS28) for RA and Western Ontario and McMaster Universities (WOMAC) for OA. Although we did not estimate the disease activity, Matsuyama et al. (20) reported that DAS-28 based on C-reactive protein levels (DAS28-CRP) were significantly higher in patients with detectable levels of IL-33 in sera than in those with undetectable levels. Despite this limitation, our study showed the similar trend of IL-33, sST2 and CRP changes after treatment. That could suggest the usefulness of IL-33 as one of the inflammatory markers such as CRP in RA. On the contrary, Mu et al. presented that the level of serum IL-33 was correlated only with the production of IgM and anti-citrullinated peptide antibody but not correlated with the score of the disease activity index (18).
It is well known that IL-6 and IL-1β are inflammatory cytokines. We discovered significant positive relationships between IL-33 and IL-1β levels, and IL-33 and IL-6 levels in the RA sera. Actually, Xu et al. (15) showed that IL-33 increases the levels of IL-1β, IL-6, IL-13, and chemokines produced in vitro by bone marrow-derived mast cells from wild-type but not from ST2-/- DBA/1 mice. Palmer et al. (14) reported that an anti-ST2 blocking antibody inhibited IL-33-induced IL-6 secretion. These results suggest that increased IL-33 leads to upregulation of inflammatory cytokines such as IL-1β and IL-6 in patients with RA. Therefore, we think that serum level of IL-33 could partially reflect the RA disease activity more than CRP alone does. Certainly, further research should be performed to ascertain the utility of IL-33 as biomarker for assessing disease activity in patient with RA.
In contrast to RA, we failed to find any significant correlation between IL-33 and IL-6 or IL-1β in sera of OA. As showed in Fig. 1A, sera of the OA showed elevated level of IL-33 comparable to that of RA. So, it is plausible that the levels of other cytokines that increase IL-33 production, which we did not measure, might be increased in the patients with OA.
In our study, the serum level of sST2 was higher in patients with RA than OA, but the concentration of sST2 in synovial fluid did not differ significantly. The comparable concentration of sST2 in OA synovial fluid to that of RA suggested the possibility of relatively higher inflammatory milieu in RA synovium, judging on the fact that sST2 acts as a decoy receptor that prevent the interaction of ST2L with IL-33. To our knowledge, this is the first report to examine sST2 levels in sera and synovial fluids from RA patients and to compare these values with those in OA patients and healthy controls. Kuroiwa et al. measured serum sST2 levels in patients with autoimmune diseases including SLE, RA, systemic sclerosis, Wegener's granulomatosis, and Behcet's disease (12). However, they did not compare the sST2 levels of serums nor that of synovial fluids from patients with these different autoimmune rheumatic diseases and healthy controls.
sST2 is secreted by mouse fibroblasts or macrophages or by human monocytes stimulated with proinflammatory cytokines and anti-CD3-activated Th2 clones (8). Therefore, it is possible that the inflammatory milieu is responsible for the increased level of sST2 in synovial fluid. Our present data also showed that serum sST2 level as well as IL-33 decreased following DMARDs therapy in treatment-naïve RA patients. This observation suggests that the inflammatory state is involved in the production of sST2 in patients with RA.
We found that ST2L was expressed in the RA synovium but we detected no ST2L expression in the OA synovium. The expression of ST2L is restricted to the surface of Th2 cell and mast cells (3), and is implicated in regulating Th2-associated immune responses, although ST2L can also promote Th1-type responses under certain conditions (21). The regulation of ST2L expression can be influenced by surrounding mediators. For example, IL-4 enhances and IFN-γ suppresses ST2L expression on Th2 cells (22). Although RA is considered to be mediated by Th1/Th17 cells, the expression of IL-4 is significantly elevated early in RA (23). Liew et al. (24) suggested that in early RA, IL-33 produced by synovial fibroblasts and synovial endothelial cells induces a Th2-type response.
The infiltration and activation of mast cells in the synovium are involved in the pathogenesis of RA (15). Liew et al. (24) also proposed that in established RA, IL-33 causes mast cell-mediated inflammation, which amplifies the Th17 cell response. In addition to mast cell, recent report (25) elucidated that IL-33 receptor was also expressed in synovial tissue, macrophage and activated neutrophil and IL-33 induced neutrophil migration by activating synoviocytes and macrophage in animal model. In that study, it was also showed that anti-TNF alpha therapy prevented IL-33-induced neutrophil migration in patients with RA. The RA patients included in our study had established RA, and we hypothesized that the increased expression of ST2L in the RA synovium would be caused by increased infiltration of mast cells or neutrophils. However, in this study we did not confirm the phenotype of cells expressing ST2L.
Whereas ST2L mediates the inflammatory effect of IL-33, sST2 has immunosuppressive activity by acting as a decoy receptor for IL-33 or has a direct anti-inflammatory action (8). Therefore, increased expression of sST2 could be a physiological mechanism to suppress the damaging inflammatory responses induced by IL-33. An anti-inflammatory action of the membrane-bound ST2 has been also suggested (26).
Although recombinant pro-IL-33 is cleaved by recombinant caspase-1 in vitro (3), the in vivo role of caspase-1 in the cleavage of pro-IL-33 remains controversial (27). There are many controversial results about IL-33 production from various kinds of cells. Actually, caspase-1-, and caspase-8-independent IL-33 production by macrophage and mast cells was also showed in recent report (28). And although pro-IL-33 was demonstrated to show biologic activity in inducing mast cell activation, cleavage of pro-IL-33 by caspase-1 in vitro resulted in loss of that biologic activity in a few reports (29). Concerning about the conversion of IL-33, it is awkward to conclude circulating IL-33 itself is biologically active.
To define the role of the IL-33/ST2 system in the pathogenesis of a disease, it is necessary to examine the expression of IL-33, sST2, and ST2L simultaneously. To our knowledge, our study is the first to measure serum IL-33 and sST2 levels simultaneously in patients with RA and OA.
In conclusion, this study demonstrated that IL-33 was significantly elevated in the sera and synovial fluids of RA and that serum level of IL-33 correlated with that of IL-1β and IL-6. The serum concentration of sST2 was also higher in RA than in healthy controls. Serum IL-33 and sST2 levels decreased together with CRP, after DMARDs therapy in patients with treatment-naïve RA. Our results suggest that IL-33 is involved in the pathogenesis of RA and may reflect the degree of inflammation in patients with RA. Further studies are needed to define the role of elevated sST2 level in patients with RA.
Figures and Tables
Fig. 1
Interleukin 33 (IL-33) levels in sera (A) or synovial fluids (B) from the patients with OA and RA comparing to HC. HC, healthy controls; OA, osteoarthritis; RA, rheumatoid arthritis. Symbols represent individual samples.

Fig. 2
The expression of IL-33 and ST2 in the synovium of RA and OA. Immunohistochemical staining of RA synovium and OA synovium. RA, rheumatoid arthritis; OA, osteoarthritis. Data are representative of 5 RA and 2 OA independent samples that yielded similar results.
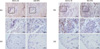
Fig. 3
The serum concentration of IL-33, sST2 and CRP before and after DMARDs therapy in patients with treatment-naïve RA. RA, rheumatoid arthritis; CRP, C-reactive protein; DMARDs, disease-modifying antirheumatic drugs.

AUTHOR SUMMARY
Measurement of Interleukin-33 (IL-33) and IL-33 Receptors (sST2 and ST2L) in Patients with Rheumatoid Arthritis
Yeon-Sik Hong, Su-Jin Moon, Young-Bin Joo, Chan-Hong Jeon, Mi-La Cho, Ji Hyeon Ju, Hye-Jwa Oh, Yu-Jung Heo, Sung-Hwan Park, Ho-Youn Kim and Jun-Ki Min
This study was conducted to determine the levels of IL-33 and sST2 in sera and synovial fluids from patients with rheumatoid arthritis (RA), osteoarthritis (OA), and healthy controls (HC). Mean levels of IL-33 and sST2 were higher in sera of RA patients than HC. And IL-33 concentration of synovial fluid was higher in RA than OA. On the other hand, synovial fluid level of sST2 did not differ between RA and OA. The serum levels of IL-33 and sST2 decreased together with C-reactive protein, after treatment with disease-modifying antirheumatic drugs in treatment-naive RA patients. Our results suggests that IL-33 is involved in the pathogenesis of RA and may reflect the degree of inflammatory condition in RA.
References
1. Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009. 384:105–109.
2. Xu D, Jiang HR, Li Y, Pushparaj PN, Kurowska-Stolarska M, Leung BP, Mu R, Tay HK, McKenzie AN, McInnes IB, Melendez AJ, Liew FY. IL-33 exacerbates autoantibody-induced arthritis. J Immunol. 2010. 184:2620–2626.
3. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005. 23:479–490.
4. Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM. Interleukin-33-cytokine of dual function or novel alarmin? Trends Immunol. 2009. 30:227–233.
5. Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008. 88:1245–1253.
6. Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007. 40:216–225.
7. Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009. 39:1046–1055.
8. Trajkovic V, Sweet MJ, Xu D. T1/ST2: an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004. 15:87–95.
9. Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, Tominaga S. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun. 2001. 285:1377–1383.
10. Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004. 173:145–150.
11. Verri WA Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A. 2008. 105:2723–2728.
12. Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001. 284:1104–1108.
13. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Gunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31:315–324.
14. Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009. 60:738–749.
15. Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008. 105:10913–10918.
16. Cavanagh LL, Boyce A, Smith L, Padmanabha J, Filgueira L, Pietschmann P, Thomas R. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res Ther. 2005. 7:R230–R240.
17. Kinne RW, Bräuer R, Stuhlmüller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000. 2:189–202.
18. Mu R, Huang HQ, Li YH, Li C, Ye H, Li ZG. Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J Rheumatol. 2010. 37:2006–2013.
19. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011. 7:33–42.
20. Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, Nagatani K, Nagashima T, Hayakawa M, Iwamoto M, Yoshio T, Tominaga S, Minota S. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J Rheumatol. 2010. 37:18–25.
21. Smithgall MD, Comeau MR, Yoon BP, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008. 20:1019–1030.
22. Carter RW, Sweet MJ, Xu D, Klemenz R, Liew FY, Chan WL. Regulation of ST2L expression on T helper (Th) type 2 cells. Eur J Immunol. 2001. 31:2979–2985.
23. Raza K, Falciani F, Curnow SJ, Ross EJ, Lee CY, Akbar AN, Lord JM, Gordon C, Buckley CD, Salmon M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005. 7:R784–R795.
24. Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010. 10:103–110.
25. Verri WA Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, Oliveira FR, Teixeira MM, Silva JS, McInnes IB, Ferreira SH, Louzada-Junior P, Liew FY, Cunha FQ. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010. 69:1697–1703.
26. Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004. 5:373–379.
27. Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007. 104:282–287.
28. Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S. Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J Immunol. 2009. 183:7890–7897.
29. Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009. 106:9021–9026.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download