Abstract
The association of hematological malignancies with a mediastinal germ cell tumor (GCT) is very rare. We report one case of a young adult male with primary mediastinal GCT who subsequently developed acute megakaryoblastic leukemia involving isochromosome (12p). A 25-yr-old man had been diagnosed with a mediastinal GCT and underwent surgical resection and adjuvant chemotherapy. At 1 week after the last cycle of chemotherapy, his peripheral blood showed leukocytosis with blasts. A bone marrow study confirmed the acute megakaryoblastic leukemia. A cytogenetic study revealed a complex karyotype with i(12p). Although additional chemotherapy was administered, the patient could not attain remission and died of septic shock. This case was definitely distinct from therapy-related secondary leukemia in terms of clinical, morphologic, and cytogenetic features. To our knowledge, this is the first case report of a patient with mediastinal GCT subsequently developing acute megakaryoblastic leukemia involving i(12p) in Korea.
Germ cell tumors (GCTs) account for 2% of human malignancies, but are the most common tumors in males 15-35 yr old (1). Mediastinal GCTs occur predominantly within the anterior mediastinum, which account for 1%-4% of mediastinal tumors, and that have different clinical characteristics from testicular GCTs (2, 3). The association between hematological malignancies and mediastinal GCT was first reported in 1983 (4) and more than 50 cases have been published since (5-9). Most often, the megakaryocytic lineage of hematopoiesis is involved in hematologic malignancy, resulting in acute megakaryoblastic leukemia, myelodysplsia with abnormal megakaryocytes, or idiopathic thrombocytopenia, essential thrombocythemia. Other hematologic diagnoses included acute lymphocytic or acute myeloid leukemia (AML) and, in rare cases, malignant histiocytosis or systemic mastocytosis (4-7, 9). A total of 64 cases of hematologic malignancies with mediastinal GCT cases have been published (1, 4-9) and only one case has reported bone marrow involvement in a mediastinal GCT case in Korea (10). Here we present a case in which a patient developed acute megakaryoblastic leukemia involving i(12p) after a primary mediastinal GCT.
A 25-yr-old man presenting with chest pain was admitted to Chung-Ang University Hospital (Seoul, Korea) because of an abnormal mass in the anterior mediastinal area. He had no remarkable past medical and familial history. He had been diagnosed with a malignant mediastinal GCT (immature teratoma 80%, embryonal carcinoma 10%, seminoma 5%, yolk sac tumor 5%) in January 2010. Peripheral blood examination showed the following: Hb level, 14.5 g/dL; leukocyte count, 10,770/µL (neutrophil 70%, lymphocyte 21%, no blasts); and platelet count, 257,000/µL. Serum alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (hCG), and lactate dehydrogenase (LDH) were 11,680 ng/mL, 0.847 mIU/mL, and 187 IU/L, respectively. After surgical resection of the tumor, the patient received four cycles of adjuvant chemotherapy with bleomycin, etoposide, and cisplatin between February and April 2010. During routine follow-up in August 2010, a persistent tumor lesion was found and the patient received three cycles of adjuvant chemotherapy with pacilitaxel, ifosfamide, and cisplatin between August and October 2010. A peripheral blood examination performed in October 2010 showed pancytopenia without blasts.
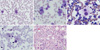
After 1 week of the last chemotherapy cycle, the patient visited the emergency room with a fever. Peripheral blood examination showed the following: Hb level, 5.9 g/dL; leukocyte count, 12,920/µL with 16% neutrophils, 22% lymphocytes, 57% blasts; 5 nucleated red cells per hundred white blood cells; and platelet count, 6,000/µL (Fig. 1A). Serum AFP and LDH levels were 234 ng/mL and 2,964 IU/L, respectively. Based on these findings, acute leukemia was suspected and a bone marrow examination was performed in November 2010. In the bone marrow aspiration, about 40.4% of all nucleated cells were blasts. The blasts were medium to large-size with round, slightly irregular nuclei and one to three nucleoli. The cytoplasm of blasts was basophilic and might show distinct bleb or pseudopod formation (Fig. 1B). The dysplastic features of a megakaryocytic lineage (e.g. micromegakaryocytes and hypolobulation of the nucleous) were found in more than 30% of 30 megarkaryocytes (Fig. 1C). Cytochemical staining showed that the cells had dot-like positivity for periodic acid-Schiff (Fig. 1D) and acid phosphatase, in contrast, negativity for myeloperoxidase, α-naphthyl acetate esterase and α-naphthyl butyrate esterase. The bone marrow biopsy showed about 90%-100% cellularity (Fig. 1E). Immunophenotyping showed positivity for CD13, CD33, CD117, MPO, CD7, CD61, and HLA-DR, and was negative for CD2, CD3, CD5, CD19, CD20, cCD22, cCD79a, CD10, CD14, and TdT. Cytogenetic examination revealed a 46,XY,add(5)(q11.2),del(10)(p11.2), -17, der(18)t(17;18) (q11.2;q21), +i(12p)[30] karyotype (Fig. 2). FISH analysis using AML1/ETO, BCR/ABL, MLL, PML/RARA, and CBFB gene probes (Vysis, Downers Grove, IL, USA) demonstrated no abnormalities. After conferring a diagnosis of acute megakaryoblastic leukemia, chemotherapy with idarubicin and ara-C was administered and the patient showed pancytopenia. The patient was then treated with mitoxantrone and ara-C, and bone marrow examination was performed that showed severe hypocellular marrow and reactive increase of histiocytes and plasma cells. Despite the additional chemotherapy, the disease not go into remission and the patient died of septic shock 1 month after the diagnosis of leukemia had been made. A summary of the clinical course is shown in Table 1.
This case concurs with previous evidence of an association between hematological malignancies and mediastinal GCTs in young males (5-9). Hematological malignancies associated with mediastinal GCTs must be distinguished from therapy-related secondary leukemia. Leukemia associated with the use of alkylating agents usually occurs after an average interval of 5-7 yr, often preceded by a preleukemic period with myelodysplasia and frequently progressing to AML with French-American-British classification (FAB) subtypes M1 or M2 (11). Topoisomerase II inhibitor-related secondary leukemia is generally diagnosed 2-3 yr after chemotherapy and most commonly exhibit FAB M4 or M5 phenotypes. While the latter type of leukemia is frequently associated with translocations of the long arm of chromosome 11 (11q23) (12-15), leukemia that appears after treatment with alkylating agents displays alterations in chromosome 5 or 7 in a high proportion of patients (60%-90%) (5).
In contrast, leukemia associated with mediastinal germ cell tumors appears to have no consistent cytogenetic abnormalities (9). However, in some cases, immunohistochemical and cytogenetic evidence, especially presence of i(12p) chromosome, had previously suggested the clonal relationship between the mediastinal GCTs and the hematologic malignancy (16). Also, two cases of leukemia have been reported in which i(12p) was found and both of these leukemia patients had mediastinal GCTs (17). The most common karyotypic abnormality in a mediastinal GCT and in the associated leukemic blasts was i(12p), suggested that i(12p) was might be a potential predictive marker for development of acute megakaryoblastic leukemia. Although our patient was treated with an alkylating agent (ifosfamide), the interval period between mediastinal GCT and the development of acute leukemia was only 10 months, the leukemia phenotype expressed in bone marrow and peripheral blood was that of acute megakaryoblastic leukemia, and a characteristic chromosomal abnormality i(12p) was also found in this case. Therefore, the clinical, morphologic, and cytogenetic features of the leukemia in our case are definitely different from therapy-related acute leukemia although the patient received seven cycles of chemotherapy.
The most possible explanation for the association between mediastinal GCTs and acute megakaryoblastic leukemia was suggested by Nichols et al. (9) who found that hematologic neoplasm is a consequence of multipotential differentiation of malignant germ cells. Presumptive corroboration of this theory is provided by the finding of i(12p) as a marker chromosome in the cytogenetic analysis of bone marrow in a patient with a mediastinal GCT and leukemia.
Hematologic neoplasms with primary mediastinal GCT possess a very aggressive clinical course. Patients tend to die before treatment, do not respond to anti-leukemic therapy, or achieve only short remissions. Allogenic bone marrow transplantation may be the only curative strategy despite one report with two patients who failed to respond to this treatment (5). However, a subgroup of patients with platelet disorders seemed to have a slightly better prognosis (4, 6). The patient in our case also showed very aggressive clinical course and little response to chemotherapy. His survival period after AML diagnosis was only 2 months.
There has been one report showing and association between mediastium and hematological diseases in Korea which presented a case of a primary mediastinal GCT with bone marrow involvement (10). However, this case did not present either acute megakaryoblastic leukemia or leukemia involving i(12p); rather, this group reported acute undifferentiated leukemia with trisomy 8 and concurrent mediastinal GCT. It remains unclear why some patients with non-seminomatous mediastinal GCTs develop a hematologic disorder whereas the majority of patients are unaffected. Based on cytogenetic findings (high incidence of i[12p]) and there is a short interval between the treatment of GCT and being diagnosed with leukemia, no relationship apparently exists between the treatment of GCTs and the development of leukemia (5).
This is the first report of a patient with a mediastinal GCT who subsequently developed acute megakaryoblastic leukemia with i(12p) in Korea. Future studies on pathogenesis of the association between these diseases are needed. Furthermore, improved strategies for treating hematological disorders in patients with mediastinal GCTs should be another area of further investigation.
Figures and Tables
Fig. 1
(A) Peripheral blood smear showing blasts (57% of nucleated cells). (B) Bone marrow smear revealed many medium-sized myeloblasts (about 40.4% of all nucleated cells). (C) Dysplasic megakaryocyte. (D) PAS positivity (granular pattern). Wright-Giemsa stain, × 1,000 magnification. (E) Bone marrow biopsy showing compact marrow with increased dysplastic megakaryocytes (H&E, × 400 magnification).
References
1. Ikdahl T, Josefsen D, Jakobsen E, Delabie J, Fosså SD. Concurrent mediastinal germ-cell tumour and haematological malignancy: case report and short review of literature. Acta Oncol. 2008. 47:466–469.
2. Takeda S, Miyoshi S, Ohta M, Minami M, Masaoka A, Matsuda H. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer. 2003. 97:367–376.
3. Weidner N. Germ-cell tumors of the mediastinum. Semin Diagn Pathol. 1999. 16:42–50.
4. Garnick MB, Griffin JD. Idiopathic thrombocytopenia in association with extragonadal germ cell cancer. Ann Intern Med. 1983. 98:926–927.
5. Hartmann JT, Nichols CR, Droz JP, Horwich A, Gerl A, Fossa SD, Beyer J, Pont J, Fizazi K, Einhorn L, Kanz L, Bokemeyer C. Hematologic disorders associated with primary mediastinal nonseminomatous germ cell tumors. J Natl Cancer Inst. 2000. 92:54–61.
6. Helman LJ, Ozols RF, Longo DL. Thrombocytopenia and extragonadal germ-cell neoplasm. Ann Intern Med. 1984. 101:280.
7. Ladanyi M, Roy I. Mediastinal germ cell tumors and histiocytosis. Hum Pathol. 1988. 19:586–590.
8. Nichols CR, Hoffman R, Einhorn LH, Williams SD, Wheeler LA, Garnick MB. Hematologic malignancies associated with primary mediastinal germ-cell tumors. Ann Intern Med. 1985. 102:603–609.
9. Nichols CR, Roth BJ, Heerema N, Griep J, Tricot G. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med. 1990. 322:1425–1429.
10. Lim JG, Lee MK, Choi JR, Park Q, Song KS, Yang CH. Bone marrow involvement of primary mediastinal germ cell tumor: a case report. Korean J Clin Pathol. 1999. 19:496–499.
11. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. French-American-British (FAB) co-operative group. Proposals for the classification of the acute leukaemias. Br J Haematol. 1976. 33:451–458.
12. Bokemeyer C, Schmoll HJ. Treatment of testicular cancer and the development of secondary malignancies. J Clin Oncol. 1995. 13:283–292.
13. Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta. 1998. 1400:233–255.
14. Ratain MJ, Rowley JD. Therapy-related acute myeloid leukemia secondary to inhibitors of topoisomerase II: from the bedside to the target genes. Ann Oncol. 1992. 3:107–111.
15. Whitlock JA, Greer JP, Lukens JN. Epipodophyllotoxin-related leukemia. Identification of a new subset of secondary leukemia. Cancer. 1991. 68:600–604.
16. Brahmanday GR, Gheorghe G, Jaiyesimi IA, Orazi A, Zekman R, Parikh R, Wills SM, Einhorn LH. Primary mediastinal germ cell tumor evolving into an extramedullary acute megakaryoblastic leukemia causing cord compression. J Clin Oncol. 2008. 26:4686–4688.
17. Duncan AM. Isochromosome of chromosome 12: clinically useful marker for male germ cell tumors. J Natl Cancer Inst. 1990. 82:1433.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download