Abstract
In addition to inhibiting cyclooxygenase and prostaglandin, nonsteroidal anti-inflammatory drugs (NSAIDs) may cause gastroduodenal injuries due to reactive oxygen species produced by recruited inflammatory cells. DA-9601 is a novel antioxidant with anti-inflammatory and cyto-protective effects. This study was conducted to compare the efficacy and safety of DA-9601 with misoprostol for preventing NSAID-associated gastroduodenal injury. In this randomized, double-blind, multicenter, noninferiority trial we compared the extents of protection of gastric and duodenal mucosae by endoscopy after 4 weeks of treatment with DA-9601 60 mg or misoprostol 200 µg three times daily, in subjects with normal baseline endoscopic findings who received an NSAID twice daily for 4 weeks. A total of 266 subjects were randomized to treatment. At week 4, the gastric protection rates with DA-9601 and misoprostol were 85.1% and 95.2%, respectively; the difference between the groups was -10.1% (var = 0.001), which was shown to indicate noninferiority of DA-9601 compared to misoprostol. Adverse events were lower in the DA-9601 group, 56.4% (95% CI, 48.0%-64.8%) than in the misoprostol group, 69.2% (95% CI, 61.3%-77.0%) (P = 0.031). DA-9601 is not inferior to misoprostol for preventing NSAID-associated gastroduodenal injury, and superior to it with respect to treatment-related side effects.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used throughout the world, but can produce significant gastrointestinal (GI) complications, varying from acute microscopic gastric mucosal changes to more serious ulcer bleeding or perforation. Epidemiologic studies estimate that peptic ulcer diseases with complications such as bleeding or perforation are 3 to 5 times higher in NSAID users than in non-NSAID users (1).
One of the factors maintaining gastroduodenal mucosal integrity is prostaglandin synthesis, but, NSAIDs can deplete prostaglandin from the gastroduodenal mucosa (2). Therefore, a strategy of replacing prostaglandin in the gastric mucosa by coadministration of misoprostol has been highly effective in patients taking NSAIDs regularly (3). Another strategy is to protect the GI mucosa with acid-suppressing agents, since low intragastric pH potentiates NSAID-induced mucosal injury (4), and there is evidence that acid suppression with proton pump inhibitors (PPIs) or H2 receptor antagonists (H2RAs) is an effective strategy (5). The use of cyclooxygenase (COX)-2-selective inhibitors rather than conventional nonselective NSAIDs is a further approach to reducing GI complications in chronic NSAID users (6).
Co-prescription of a COX-2 inhibitor with a PPI is being recommended as the most effective strategy for preventing gastroduodenal ulcers in chronic NSAID users. However, due to concerns about the high cost of PPIs, the increased risk of cardiovascular complications associated with COX-2 inhibitors (7) and low compliance due to side effects of misoprostol such as diarrhea, nausea, and abdominal pain, the effectiveness of this strategy is limited in reality. Therefore, a new strategy involving lower cost and better compliance is needed in this era of prevailing chronic NSAID users.
NSAID-induced mucosal injury may occur due to the production of reactive oxygen species (ROS) by recruited leukocytes as well as inhibition of COX and reduction of prostaglandin synthesis. Therefore, inhibiting NSAID-inducible ROS production could theoretically prevent NSAID-induced mucosal injury. This idea is supported by an animal study showing that an antioxidant with anti-inflammatory activity promoted gastric ulcer healing (8). Also, a formulated ethanol extract of Artemisia asiatica was reported to restore the epithelial migration and actin disruption induced by hydrogen peroxide on in vitro study, indicating its antioxidant effect that repairs the gastric mucosa (9). DA-9601 (Stillen™) is a phytopharmaceutical derived from A. asiatica that has anti-inflammatory and anti-oxidant properties attributable to inhibition of ERK 1/2 activation (10). These anti-inflammatory and cytoprotective activities of DA-9601 have been shown to be against experimentally-induced damages in the liver and pancreas as well as the GI tract (11-13). In animal studies, it had an anti-oxidative effect on reflux esophagitis (14) and attenuated gastric hemorrhagic lesions induced by alcohol (15). Furthermore, a clinical trial demonstrated that endoscopy-proven erosive gastritides were not only effectively treated but also well-tolerated by DA-9601 (16). Therefore, DA-9601 is expected to be able to become a highly attractive option for preventing GI injury by noxious stimuli such as NSAIDs.
The purpose of the present study was to demonstrate the non-inferiority of DA-9601 compared with misoprostol for preventing NSAID-induced gastroduodenal injury in terms of efficacy and safety. Misoprostol has been proven to be effective against NSAID-induced GI toxicity and was therefore selected as an active control drug in this head-to-head study. We evaluated esophagogastroduodenoscopic (EGD) findings in healthy volunteers before and after taking an NSAID twice daily for 4 weeks together with either misoprostol or DA-9601.
The study was a randomized, double-blind, multicenter non-inferiority trial. Subjects were requested to visit each study center three times; on the first visit for screening (day-7 to day-1), medical histories, previous medications, and symptoms were recorded, and physical examinations and clinical laboratory tests were performed. On the second visit (day 0), all the subjects underwent baseline EGD. Follow up EGD was performed on the final visit (4 weeks after day 0).
The eligible subjects were randomized to one of the study medications: DA-9601 or misoprostol, according to a computer-generated randomization schedule in balanced blocks site by site. The subjects were assigned sequential allocation numbers at each site and the medications were presented identical-appearing tablets to maintain blinding.
Subjects received either misoprostol 200 µg t.i.d. or DA-9601 60 mg t.i.d. after meals for 4 weeks. During the same period, they were instructed to take an NSAID (aceclofenac, 100 mg) b.i.d. after meals in the morning and evening.
Eligible subjects were healthy volunteers aged 18 to 65 yr who had normal baseline endoscopy findings with no erosions and ulcers i.e.- grading scores of 0 to 1 (Table 1).
Exclusion criteria included any of the following: gastric or duodenal ulcer within 30 days of the first dose of study medication, previous gastrointestinal surgery, alcoholism, history of hypersensitivity to any drug, drug dependency, any use (≥ 5 days) of NSAIDs, H2 RA, PPIs, sucralfate, misoprostol, stillen, anticoagulants, triamterene, corticosteroids, antineoplastic drugs, and cyclophosphamide or methotrexate within 30 days of the first dose of study medication. Individuals with the following conditions were also excluded: major hematological, renal, cardiac, pulmonary, and hepatic abnormalities; thrombotic disorders; consumption coagulopathy and psychiatric disorders. Women of child-bearing potential had adequate contraception.
Each subject underwent an EGD at baseline and after 4 week at each study site. The primary end point was the protec-tion of gastric mucosal lesions at week 4 based on endoscopy scores ranging from 0 to 5, with 5 representing ulcers (Table 1). The primary analysis was the percentage of subjects who were protected (defined as endoscopy scores of 0-1) after 4 weeks of study medication. Secondary end points were protection from endoscopic duodenal mucosal lesions and the observed number of duodenal ulcers on week 4. Every day subjects used a telephone-based interactive voice response system to answer the question whether they had taken their NSAID as prescribed.
All adverse events (AEs) reported by the subjects or observed by the investigators were recorded on all scheduled and unscheduled visits. These included adverse drug reactions, illnesses with onset during the study, any GI symptoms, and clinically significant changes in physical examination findings, vital signs or electrocardiograms.
The gastric endoscopic protection rate was set at 67% and the margin of non-inferiority, at 17%, based on a previous study (17). The margin of non-inferiority corresponds to 40% of the difference in gastric endoscopic efficacy rate between misoprostol and placebo in the previous study. This percentage is in accordance with the commonly accepted standard in non-inferiority tests. The number of subjects was determined assuming the level of significance α to be 0.05 and 80% statistical power for the test. Our target sample size was computed as 190 subjects; 95 subjects per group. Assuming an average dropout rate of 20%, 238 subjects (119 subjects per group) were recruited.
Demographic and baseline characteristics were summarized descriptively for all the participating subjects. The Wilcoxon rank sum test was used to evaluate the statistical significance of intergroup differences in continuous data, and the chi-square test, for categorical data.
All efficacy analyses were performed on the intention-to-treat (ITT) population and per protocol (PP) population. For the primary efficacy endpoint of gastric endoscopic protection rate, the lower limit of the one-sided 95% confidence interval (CI) of difference in rate between DA-9601 and misoprostol was computed using a Meta analysis that was adjusted for site effect as block (25). The gastric endoscopic efficacy of DA-9601 (test group) could be considered non-inferior to misoprostol (control group) if the lower limit of the one-sided 95% confidence interval (CI) was equal to or greater than -17. Fisher's exact test was used to assess differences of duodenal endoscopic protection rates and incidence rates of duodenal ulcers between the two groups. For adverse events, 95% CIs for the frequency (%) of occurrence and number of cases of adverse events were calculated for subjects who experienced one or more adverse drug reactions. Inter-group comparisons were conducted using the chi-square test.
Approval for the study was obtained from the institutional review board of each of the nine participating Korean centers including Inje University Busan Paik Hospital, College of Medicine (IRB approval number: 05-62) and it was conducted in compliance with good clinical practice, including ICH guidelines. All subjects provided written informed consent before enrollment.
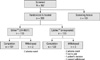
A total of 367 subjects were enrolled from nine Korean centers, of whom 266 entered and 259 completed the study (Fig. 1). Of the 266 subjects randomized, two of the DA-9601 group discontinued the study medication due to adverse events and five of the misoprostol group were not included because of adverse events, loss to visits and patients' decision.
Baseline demographics and characteristics of the study subjects are summarized in Table 2. The mean ages of subjects in the two groups were 25 and 24 yr respectively. 43.6% of the subjects were female. The treatment groups were well balanced at baseline, including age, gender, height, weight, smoking status and alcohol consumption.
The PP assay population comprised 259 patients (131 in the DA-9601 group and 128 in the misoprostol group). The gastric endoscopic protection rates were 87.3% in the DA-9601 group and 95.6% in the misoprostol group. The difference in gastric endoscopic protection rates between the two groups was -8.3% (var = 0.001). The lower limit of the 95% CI was -13.9%; this is higher than -17%, indicating that DA-9601 is not inferior to misoprostol (Table 3).
In secondary analyses, Fisher's exact tests showed that the duodenal endoscopic protection rates were 97.7% (130/133, 95% CI, 93.6%-99.5%) in the DA-9601 group and 96.2% (128/133, 95% CI 91.4%-98.8%) in the misoprostol group, indicating that there was no difference between the two groups (P = 0.722) (Table 4). One subject in the DA-9601 group developed a duodenal ulcer by week 4, while there was no incidence of duodenal ulcers in the misoprostol group. The incidence of duodenal ulcers was 0.76% in the DA-9601 group, not different from that in the misoprostol group (P = 1.00). Table 4 presents the ITT analyses demonstrating the absence of statistically significant differences between the two study populations in the secondary end points.
The overall percentage of subjects with drug-related adverse effects (AEs) was 62.8% (75/133 in the DA-9601 group and 92/133 in the misoprostol group). The incidence rates of AEs were 56.4% (95% CI, 48.0%-64.8%) in the DA-9601 group and 69.2% (95% CI, 61.3%-77.0%) in the misoprostol group (P = 0.031) (Table 5).
The most common AE observed was diarrhea, reported in 31 individuals (23.3%) in the DA-9601 group and 35 (26.3%) in the misoprostol group. Percentage occurrences of other AEs in the DA-9601 and misoprostol groups were: abdominal distension, 21 (15.8%) in the DA-9601 group vs 42 (31.9%) in the misoprostol group, upper abdominal pain 22 (16.5%) vs 27 (20.3%), abdominal pain 14 (10.5%) vs 21 (15.8%), and nausea 14 (10.5%) vs 19 (14.3%).
Each score for GI symptoms was assessed by the Friedman test (Table 6). The differences between symptom measurements at the outset and after four weeks were not statistically significant in the DA-9601 group (P = 0.40), but there was a significant difference in the misoprostol group (P = 0.00). The difference between the GI symptom scores in week 0 and week 4 was significantly lower in the DA-9601 group by the Wilcoxon rank sum test (0.32 in the DA-9601 vs 1.01 in the misoprostol group, P = 0.029).
Our study was designed to assess the non-inferiority of DA-9601 compared to misoprostol for preventing NSAID-associated gastric or duodenal injuries. In this randomized, double blind, multicenter trial performed in healthy volunteers who took an NSAID twice daily for 4 weeks, DA-9601 was not inferior to misoprostol in protecting from endoscopic gastroduodenal mucosal lesions and superior to misoprostol with respect to the development of AEs.
The protective effects of misoprostol are related to replacement of the prostaglandin whose production is inhibited by NSAIDs. Antisecretory agents such as PPIs and H2RAs attenuate the acidic environment in which condition NSAIDs are inclined to evoke GI toxicities. The preventive effect of DA-9601 seems to be due to its antioxidative and cytoprotective actions in the GI mucosa (9, 10). Noxious agents such as NSAIDs commonly cause oxidative injury to the upper GI mucosa. Eupatilin, a major component of DA-9601, was shown to protect H2O2-induced gastric epithelial damage and to inhibit FeSO4-induced ROS production, which is accompanied by reduced oxidative-driven gene expression (9). Production of an important inflammatory mediators, TNF-α, was inhibited by DA-9601 in gastric epithelial cells via modulation of the p38 kinase and NF-κB-dependent pathways (18). Suppression of inflammatory mediators by DA-9601 via the NF-κB pathway was also noted in an animal model of allergic asthma (19), and anti-inflammatory and cytoprotective effects of DA-9601 have also been observed in situations involving experimentally-induced hepatic or pancreatic damage. DA-9601 attenuated the acute severe pancreatitis induced by cerulean in rats (13), and intragastrically administered DA-9601 had a dose-dependent hepatoprotective effect on acetaminophen- and carbon tetrachloride-induced glutathione depletion in rats (12).
Many studies have demonstrated a gastroprotective effect of DA-9601 in animal models. Reflux esophagitis provoked by oxidative stress in rats was more significantly improved by DA-9601 in terms of histopathology than by antisecretory medications such as H2RA (14). In rats with ethanol-induced gastric mucosal damage, DA-9601 reduced gastric hemorrhagic lesions and inhibited lipid peroxidation by opposing the induction of xanthine oxidase by alcohol (15). DA-9601 and omeprazole also had a synergistic protective effect on peptic ulcers and gastroesophageal reflux disease in alcohol-, and indomethacin-induced animal models (20). In addition to animal studies, a clinical trial involving 512 patients with erosive gastritis showed that DA-9601 was more effective in endoscopically-demonstrated healing of the gastric mucosa than cetraxate, as well as having an excellent adverse effect profile (16). Since 2002, DA-9601 has been widely used for the treatment of gastritides throughout Korea, and its sales have grown rapidly to US$78 million equivalent in 2010.
In addition to its mucosal protective effects, we found that DA-9601 was better tolerated by the study subjects than misoprostol. Many studies have mentioned a significant problem of compliance with misoprostol (21) attributable to adverse side effects such as diarrhea, nausea, and abdominal pain. AEs reported in our study subjects were similar to the previously known side effects of misoprostol. These GI symptoms also occurred in the DA-9601 group though less frequenctly. Therefore, DA-9601 is expected to achieve relatively good compliance in patients who continue to take NSAIDs.
The upper GI toxicity of NSAIDs increases with high dose of NSAIDs, use of multiple NSAIDs, and long-term use of NSAIDs. Risk factors of NSAID-associated GI mucosal injury include old age, prior history of peptic ulcer diseases, and concurrent therapy with warfarin or glucocorticoids. In a study in the general population, traditional nonselective NSAIDs were shown to be associated with a 3- to 4-fold increase of upper GI complications compared to 2- to 3-fold increase with COX-2 inhibitors (22). We studied a group of healthy volunteers, and it is unclear whether our results would be applicable to those with high risk factors. Our results are valuable when considering the increasing number of healthy individuals using NSAIDs on account of musculoskeletal traumas such as sports and car accidents, and future studies should clarify the effect of DA-9601 in high risk patients. Whether ulcer prevention could be maintained with long-term DA-9601 treatment in patients who require continued NSAIDs therapy remains to be determined.
Studies have shown that misoprostol reduces the risk of NSAID-associated gastric and duodenal ulcers compared to placebo (23, 24). The ulcer-preventive effects of misoprostol have been reported to be at least similar to, and mostly superior to double-dose of H2RA or standard dose of PPI (25, 26). However, most studies comparing it directly with antisecretory agents such as H2RA or PPIs indicate that compliance is lower in the case of misoprostol and that its efficacy is almost the same as the other treatments in preventing duodenal ulcers (25-28). These findings are in agreement with our results showing that subjects in the misoprostol group complained of significantly more AEs and that efficacy did not differ between the two groups. Taken together, we speculate that the efficacy of DA-9601 in preventing NSAID-associated gastroduodenal injury is comparable to that of antisecretory agents, although direct comparisons are needed to confirm this.
For optimal GI safety in patients using NSAIDs chronically, the administration of a COX-2 inhibitor together with a PPI is recommended (29). However, this strategy is very costly and raises concerns regarding potential cardiovascular complications. Furthermore, apart from their low cost to benefit ratios, PPIs have potential side effects such as osteoporotic fracture, infection, neoplasia, and interaction with clopidogrel (30). Our results suggest that a new agent with an acceptable cost/benefit ratio may be at hand for preventing NSAIDs-associated gastroduodenal injury. This new agent, DA-9601, offers similar efficacy and superior safety to misoprostol.
In conclusion, our study indicates that DA-9601 is not inferior to misoprostol in preventing NSAIDS-associated gastric and duodenal mucosal injury and that AEs are less frequent with DA-9601 than with misoprostol. Based on these results, we recommend that patients who have to continue NSAID therapy be treated with DA-9601 in order to prevent GI toxicity from NSAIDs.
Figures and Tables
Fig. 1
Patient disposition. Cumulative number of dropouts and medical reasons for withdrawal from the study.
AUTHOR SUMMARY
Prevention of NSAID-Associated Gastroduodenal Injury in Healthy Volunteers-A Randomized, Double-Blind, Multicenter Study Comparing DA-9601 with Misoprostol
Kang Nyeong Lee, Oh Young Lee, Myung-Gyu Choi, Seok Reyol Choi, Dong Ho Lee, Yong Chan Lee, Tae Nyeun Kim, Suck Chei Choi, Jong Sun Rew and Sang-Young Seol
Chronic application of NSAIDs often causes gastrointestinal complications. This study was designed to assess the efficacy and safety of a novel antioxidant, DA-9601, compared to an efficacy-proven drug, misoprostol for preventing gastroduodenal injury. Healthy volunteers took an NSAID, aceclofenac, with either DA-9601 or misoprostol for 4 weeks and returned for follow up endoscopy. Our results show that DA-9601 is not inferior to misoprostol in the endoscopic gastroduodenal protection rates and that side-effect profiles of DA-9601 were better than misoprostol. Based on these results, we recommend that patients who have to continue NSAID therapy can be treated with DA-9601 to prevent GI toxicity.
References
1. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994. 343:769–772.
2. Scheiman JM. Pathogenesis of gastroduodenal injury due to nonsteroidal antiinflammatory drugs: implications for prevention and therapy. Semin Arthritis Rheum. 1992. 21:201–210.
3. Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, Geis GS. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995. 123:241–249.
4. Schoen RT, Vender RJ. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Am J Med. 1989. 86:449–458.
5. Agrawal NM, Campbell DR, Safdi MA, Lukasik NL, Huang B, Haber MM. NSAID-Associated Gastric Ulcer Study Group. Superiority of lansoprazole vs ranitidine in healing nonsteroidal anti-inflammatory drug-associated gastric ulcers: results of a double-blind, randomized, multicenter study. Arch Intern Med. 2000. 160:1455–1461.
6. Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Matchaba P, Gimona A, Hawkey CJ. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomized controlled trial. Lancet. 2004. 364:665–674.
7. Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomized trials. BMJ. 2006. 332:1302–1308.
8. Kang JM, Kim N, Kim B, Kim JH, Lee BY, Park JH, Lee MK, Lee HS, Kim JS, Jung HC, Song IS. Enhancement of gastric ulcer healing and angiogenesis by cochinchina Momordica seed extract in rats. J Korean Med Sci. 2010. 25:875–881.
9. Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ, Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, Kim SW, Jun CD. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008. 25:1355–1364.
10. Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem Pharmacol. 2004. 68:1081–1087.
11. Ahn BO, Ko KH, Oh TY, Cho H, Kim WB, Lee KJ, Cho SW, Hahm KB. Efficacy of use of colonoscopy in dextran sulfate sodium induced ulcerative colitis in rats: the evaluation of the effects of antioxidant by colonoscopy. Int J Colorectal Dis. 2001. 16:174–181.
12. Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharm Res. 1998. 21:508–513.
13. Hahm KB, Kim JH, You BM, Kim YS, Cho SW, Yim H, Ahn BO, Kim WB. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998. 17:153–157.
14. Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Lee KM, Hahm KB. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001. 49:364–371.
15. Huh K, Kwon TH, Shin US, Kim WB, Ahn BO, Oh TY, Kim JA. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003. 88:269–273.
16. Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004. 10:2379–2382.
17. Lanza FL, Fakouhi D, Rubin A, Davis RE, Rack MF, Nissen C, Geis S. A double-blind placebo-controlled comparison of the efficacy and safety of 50, 100, and 200 micrograms of misoprostol QID in the prevention of ibuprofen-induced gastric and duodenal mucosal lesions and symptoms. Am J Gastroenterol. 1989. 84:633–636.
18. Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, Lee WJ, Park JS, Shin CY, Oh TY, Jun CD. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006. 12:4850–4858.
19. Kim JY, Kim DY, Lee YS, Lee BK, Lee KH, Ro JY. DA-9601, Artemisia asiatica herbal extract, ameliorates airway inflammation of allergic asthma in mice. Mol Cells. 2006. 22:104–112.
20. Kim JM, Choi SM, Kim DH, Oh TY, Ahn BO, Kwon JW, Kim WB. Combined use of omeprazole and a novel antioxidative cytoprotectant for the treatment of peptic ulcer. Facilitation of ulcer healing in experimental animals. Arzneimittelforschung. 2005. 55:387–393.
21. Weaver AL, Gitlin N. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs. Arch Intern Med. 2002. 162:2248–2249.
22. García Rodríguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007. 132:498–506.
23. Agrawal NM, Caldwell J, Kivitz AJ, Weaver AL, Bocanegra TS, Ball J, Dhadda S, Hurley S, Hancock L. Comparison of the upper gastrointestinal safety of Arthrotec 75 and nabumetone in osteoarthritis patients at high risk for developing nonsteroidal anti-inflammatory drug-induced gastrointestinal ulcers. Clin Ther. 1999. 21:659–674.
24. Chan FK, Sung JJ, Ching JY, Wu JC, Lee YT, Leung WK, Hui Y, Chan LY, Lai AC, Chung SC. Randomized trial of low-dose misoprostol and naproxen vs. nabumetone to prevent recurrent upper gastrointestinal haemorrhage in users of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2001. 15:19–24.
25. Raskin JB, White RH, Jaszewski R, Korsten MA, Schubert TT, Fort JG. Misoprostol and ranitidine in the prevention of NSAID-induced ulcers: a prospective, double-blind, multicenter study. Am J Gastroenterol. 1996. 91:223–227.
26. Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL, Huang B. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002. 162:169–175.
27. Hawkey CJ, Karrasch JA, Szczepañski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1998. 338:727–734.
28. Valentini M, Cannizzaro R, Poletti M, Bortolussi R, Fracasso A, Testa V, Sozzi M, Fornasarig M, Bortoluzzi F, Grazioli I. Nonsteroidal antiinflammatory drugs for cancer pain: comparison between misoprostol and ranitidine in prevention of upper gastrointestinal damage. J Clin Oncol. 1995. 13:2637–2642.
29. Targownik LE, Metge CJ, Leung S, Chateau DG. The relative efficacies of gastroprotective strategies in chronic users of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2008. 134:937–944.
30. Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010. 139:1115–1127.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download